Skin wounds can normally heal without problem; however, some diseases or extensive damage may delay or prevent healing. Non-healing wounds represent a serious and life-threatening scenario that may require advanced therapeutic strategies. The main task of skin stem cells (SSCs) is to replace, restore, and regenerate the epidermal cells that may have been lost, damaged, or have become pathologically dysfunctional.
- skin stem cells
- non-healing wounds
- skin regeneration
- tissue engineering
- wound healing
1. Introduction
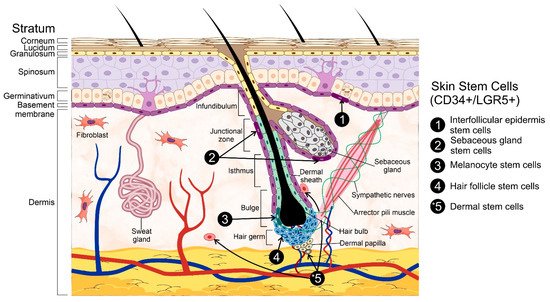
2. Skin Stem Cells and Wound Healing
2.1. Cell Signaling Pathways and SSCs
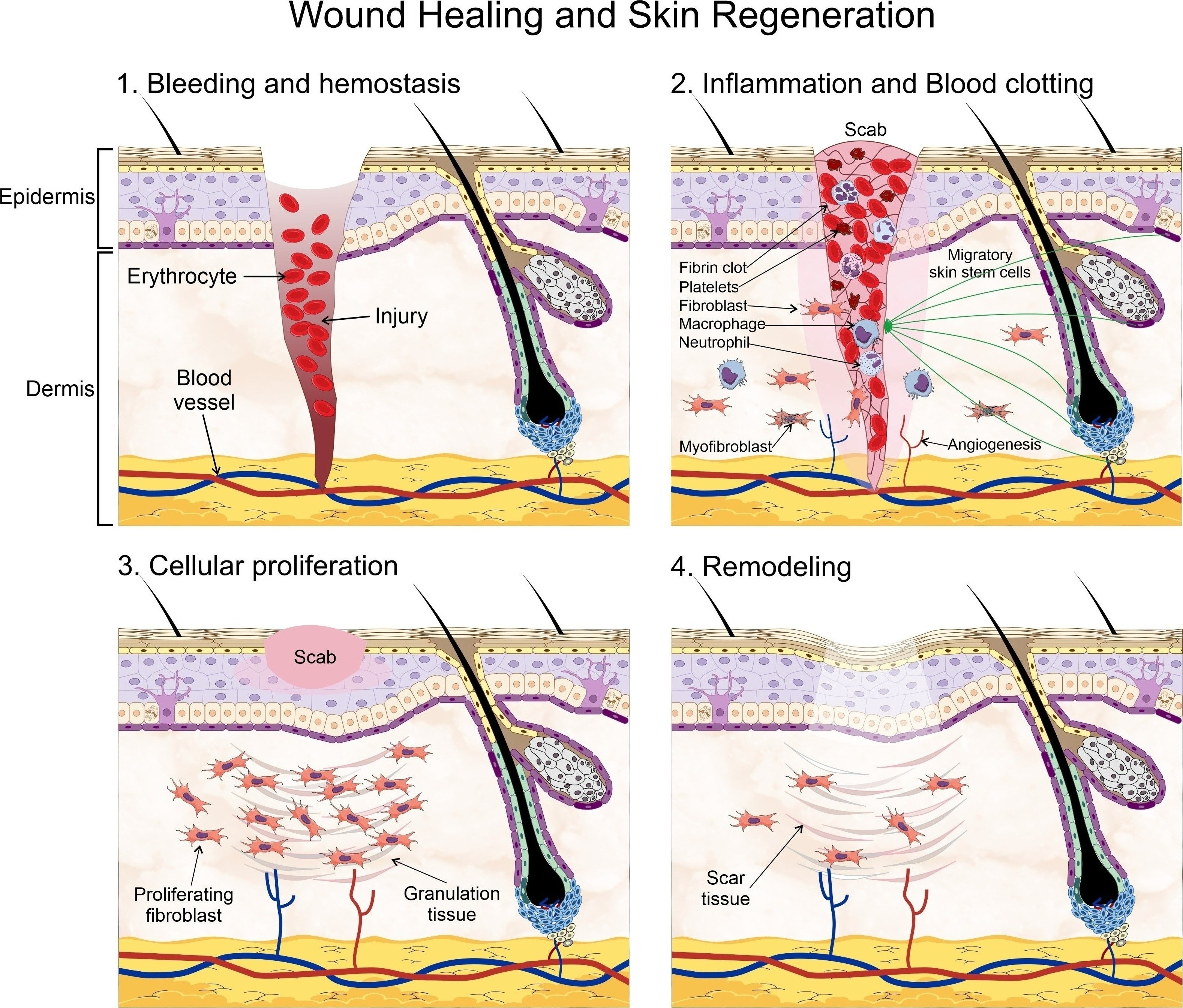
2.2. Principles of Wound Healing and Skin Regeneration
2.3. Scarless Wound Healing
2.4. Chronic Wounds
As we already know, wound healing is a complex and multi-step procedure that requires an inflammatory reaction, wound clotting, re-epithelialization, remodeling regulation, and stem cell control. In recent years, the role of SSCs in wound healing has been recognized as an integral part in the healing of diabetic wounds, as they are essential during the steps of wound closure and tissue remodeling. Therefore, the direct loss of SSCs, their impaired migration capacity, and/or faulty differentiation capacity may have a profoundly negative effect on wound healing. One of such impairing effects has been directly related with telomere stability, as it can hinder both the proliferation and migration capacity of SSCs [73]. The telomeres have been involved in a wide range of cellular processes, including ageing and cancer. Moreover, telomere maintenance is crucial for the longevity of stem cells. The upkeep of the telomeres’ length is responsibility of an enzyme known as telomerase, which is normally downregulated in somatic cells but highly expressed in both cancer and stem cells. An integral part of this enzyme is known as telomerase reverse transcriptase (TERT) [74]. TERT is involved in some types of trauma, particularly through the activation of NF-κB and autophagy [75]. In SSCs, however, TERT expression is required for normal cell proliferation and migration; further, its expression is closely associated with the activation of the Wnt/β-catenin signaling pathway [76]. The inactivation of this signaling pathway, which has been associated with the inflammatory response in diabetic ulcers, wound proliferation and remodeling [77], has also been linked with the expression of telomerase; although the precise mechanism still remains obscure.
2.5. Wound Healing Therapy
References
- Arron, S. Anatomy of the Skin and Pathophysiology of Radiation Dermatitis. In Skin Care in Radiation Oncology; Fowble, B., Yom, S.S., Yuen, F., Arron, S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 9–14. ISBN 9783319314587.
- McGrath, J.A.; Uitto, J. Anatomy and Organization of Human Skin. In Rook’s Textbook of Dermatology; Burns, T., Breathnach, S., Cox, N., Griffiths, C., Eds.; Wiley-Blackwell: Oxford, UK, 2010; pp. 1–53. ISBN 9781444317633.
- Garland, E.L. Pain Processing in the Human Nervous System. Prim. Care Clin. Off. Pract. 2012, 39, 561–571.
- Kolarsick, P.; Kolarsick, M.; Goodwin, C. Anatomy and Physiology of the Skin: Erratum. J. Dermatol. Nurses’ Assoc. 2011, 3, 366.
- Honari, G.; Maibach, H. Skin Structure and Function. In Applied Dermatotoxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–10. ISBN 9780124201309.
- Leung, Y.; Kandyba, E.; Chen, Y.-B.; Ruffins, S.; Chuong, C.-M.; Kobielak, K. Bifunctional Ectodermal Stem Cells around the Nail Display Dual Fate Homeostasis and Adaptive Wounding Response toward Nail Regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 15114–15119.
- Blanpain, C.; Fuchs, E. Epidermal Homeostasis: A Balancing Act of Stem Cells in the Skin. Nat. Rev. Mol. Cell Biol. 2009, 10, 207–217.
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2015, 4, 213–224.
- Blanpain, C.; Fuchs, E. Epidermal Stem Cells of the Skin. Ann. Rev. Cell Dev. Biol. 2006, 22, 339–373.
- Fuchs, E. Skin Stem Cells: Rising to the Surface. J. Cell Biol. 2008, 180, 273–284.
- Racila, D.; Bickenbach, J.R. Are Epidermal Stem Cells Unique with Respect to Aging? Aging 2009, 1, 746–750.
- Giangreco, A.; Qin, M.; Pintar, J.E.; Watt, F.M. Epidermal Stem Cells Are Retained in Vivo throughout Skin Aging. Aging Cell 2008, 7, 250–259.
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401.
- Psarras, S.; Beis, D.; Nikouli, S.; Tsikitis, M.; Capetanaki, Y. Three in a Box: Understanding Cardiomyocyte, Fibroblast, and Innate Immune Cell Interactions to Orchestrate Cardiac Repair Processes. Front. Cardiovasc. Med. 2019, 6, 32.
- Kizil, C.; Kyritsis, N.; Brand, M. Effects of Inflammation on Stem Cells: Together They Strive? EMBO Rep. 2015, 16, 416–426.
- Logan, C.Y.; Nusse, R. The wnt Signaling Pathway in Development and Disease. Ann. Rev. Cell Dev. Biol. 2004, 20, 781–810.
- Nusse, R. Wnt Signaling and Stem Cell Control. Cell Res. 2008, 18, 523–527.
- Clevers, H. Wnt/β-Catenin Signaling in Development and Disease. Cell 2006, 127, 469–480.
- Holland, J.D.; Klaus, A.; Garratt, A.N.; Birchmeier, W. Wnt Signaling in Stem and Cancer Stem Cells. Curr. Opin. Cell Biol. 2013, 25, 254–264.
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999.
- van Amerongen, R.; Nusse, R. Towards an Integrated View of Wnt Signaling in Development. Development 2009, 136, 3205–3214.
- Nelson, W.J. Convergence of Wnt—Catenin, and Cadherin Pathways. Science 2004, 303, 1483–1487.
- Wehrli, M.; Dougan, S.T.; Caldwell, K.; O’Keefe, L.; Schwartz, S.; Vaizel-Ohayon, D.; Schejter, E.; Tomlinson, A.; DiNardo, S. Arrow Encodes an LDL-Receptor-Related Protein Essential for Wingless Signalling. Nature 2000, 407, 527–530.
- Tamai, K.; Semenov, M.; Kato, Y.; Spokony, R.; Liu, C.; Katsuyama, Y.; Hess, F.; Saint-Jeannet, J.-P.; He, X. LDL-Receptor-Related Proteins in Wnt Signal Transduction. Nature 2000, 407, 530–535.
- Wang, Y. The Role of Frizzled3 and Frizzled6 in Neural Tube Closure and in the Planar Polarity of Inner-Ear Sensory Hair Cells. J. Neurosci. 2006, 26, 2147–2156.
- de Lau, W.; Peng, W.C.; Gros, P.; Clevers, H. The R-Spondin/Lgr5/Rnf43 Module: Regulator of Wnt Signal Strength. Genes Dev. 2014, 28, 305–316.
- Wilson, S.; Rydström, A.; Trimborn, T.; Willert, K.; Nusse, R.; Jessell, T.M.; Edlund, T. The Status of Wnt Signalling Regulates Neural and Epidermal Fates in the Chick Embryo. Nature 2001, 411, 325–330.
- Wilson, P.A.; Hemmati-Brivanlou, A. Induction of Epidermis and Inhibition of Neural Fate by Bmp-4. Nature 1995, 376, 331–333.
- Fuchs, E. Scratching the Surface of Skin Development. Nature 2007, 445, 834–842.
- Zhu, X.-J.; Liu, Y.; Dai, Z.-M.; Zhang, X.; Yang, X.; Li, Y.; Qiu, M.; Fu, J.; Hsu, W.; Chen, Y.; et al. BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin. PLoS Genet. 2014, 10, e1004687.
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell 2001, 105, 533–545.
- Niemann, C.; Owens, D.M.; Hülsken, J.; Birchmeier, W.; Watt, F.M. Expression of DeltaNLef1 in Mouse Epidermis Results in Differentiation of Hair Follicles into Squamous Epidermal Cysts and Formation of Skin Tumours. Dev. Camb. Engl. 2002, 129, 95–109.
- M’Boneko, V.; Merker, H.-J. Development and Morphology of the Periderm of Mouse Embryos (Days 9–12 of Gestation). Cells Tissues Organs 1988, 133, 325–336.
- Popp, T.; Steinritz, D.; Breit, A.; Deppe, J.; Egea, V.; Schmidt, A.; Gudermann, T.; Weber, C.; Ries, C. Wnt5a/β-Catenin Signaling Drives Calcium-Induced Differentiation of Human Primary Keratinocytes. J. Investig. Dermatol. 2014, 134, 2183–2191.
- Schmidt-Ullrich, R.; Paus, R. Molecular Principles of Hair Follicle Induction and Morphogenesis. BioEssays 2005, 27, 247–261.
- van der Veen, C.; Handjiski, B.; Paus, R.; Müller-Röver, S.; Maurer, M.; Eichmüller, S.; Ling, G.; Hofmann, U.; Foitzik, K.; Mecklenburg, L. A Comprehensive Guide for the Recognition and Classification of Distinct Stages of Hair Follicle Morphogenesis. J. Investig. Dermatol. 1999, 113, 523–532.
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal-Catenin Activity in Response to Epidermal Wnt Ligands Is Required for Fibroblast Proliferation and Hair Follicle Initiation. Development 2012, 139, 1522–1533.
- Millar, S.E. Molecular Mechanisms Regulating Hair Follicle Development. J. Investig. Dermatol. 2002, 118, 216–225.
- Richardson, R.; Slanchev, K.; Kraus, C.; Knyphausen, P.; Eming, S.; Hammerschmidt, M. Adult Zebrafish as a Model System for Cutaneous Wound-Healing Research. J. Investig. Dermatol. 2013, 133, 1655–1665.
- Seifert, A.W.; Monaghan, J.R.; Voss, S.R.; Maden, M. Skin Regeneration in Adult Axolotls: A Blueprint for Scar-Free Healing in Vertebrates. PLoS ONE 2012, 7, e32875.
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem Cells in the Hair Follicle Bulge Contribute to Wound Repair but Not to Homeostasis of the Epidermis. Nat. Med. 2005, 11, 1351–1354.
- Mascré, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohée, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct Contribution of Stem and Progenitor Cells to Epidermal Maintenance. Nature 2012, 489, 257–262.
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair Follicle Dermal Papilla Cells at a Glance. J. Cell Sci. 2011, 124, 1179–1182.
- Biernaskie, J.; Paris, M.; Morozova, O.; Fagan, B.M.; Marra, M.; Pevny, L.; Miller, F.D. SKPs Derive from Hair Follicle Precursors and Exhibit Properties of Adult Dermal Stem Cells. Cell Stem Cell 2009, 5, 610–623.
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281.
- Lichtenberger, B.M.; Mastrogiannaki, M.; Watt, F.M. Epidermal β-Catenin Activation Remodels the Dermis via Paracrine Signalling to Distinct Fibroblast Lineages. Nat. Commun. 2016, 7, 10537.
- Rinkevich, Y.; Walmsley, G.G.; Hu, M.S.; Maan, Z.N.; Newman, A.M.; Drukker, M.; Januszyk, M.; Krampitz, G.W.; Gurtner, G.C.; Lorenz, H.P.; et al. Identification and Isolation of a Dermal Lineage with Intrinsic Fibrogenic Potential. Science 2015, 348, aaa2151.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321.
- Wong, V.W.; Rustad, K.C.; Akaishi, S.; Sorkin, M.; Glotzbach, J.P.; Januszyk, M.; Nelson, E.R.; Levi, K.; Paterno, J.; Vial, I.N.; et al. Focal Adhesion Kinase Links Mechanical Force to Skin Fibrosis via Inflammatory Signaling. Nat. Med. 2012, 18, 148–152.
- Wynn, T. Cellular and Molecular Mechanisms of Fibrosis. J. Pathol. 2008, 214, 199–210.
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless Fetal Wound Healing: A Basic Science Review. Plast. Reconstr. Surg. 2010, 126, 1172–1180.
- De Souza, K.S.; Cantaruti, T.A.; Azevedo, G.M.; de Galdino, D.A.A.; Rodrigues, C.M.; Costa, R.A.; Vaz, N.M.; Carvalho, C.R. Improved Cutaneous Wound Healing after Intraperitoneal Injection of Alpha-Melanocyte-Stimulating Hormone. Exp. Dermatol. 2015, 24, 198–203.
- Singla, D.K.; Singla, R.D.; Abdelli, L.S.; Glass, C. Fibroblast Growth Factor-9 Enhances M2 Macrophage Differentiation and Attenuates Adverse Cardiac Remodeling in the Infarcted Diabetic Heart. PLoS ONE 2015, 10, e0120739.
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from Dermal Γδ T Cells Induces Hair Follicle Neogenesis after Wounding. Nat. Med. 2013, 19, 916–923.
- Morris, M.W.; Allukian, M.; Herdrich, B.J.; Caskey, R.C.; Zgheib, C.; Xu, J.; Dorsett-Martin, W.; Mitchell, M.E.; Liechty, K.W. Modulation of the Inflammatory Response by Increasing Fetal Wound Size or Interleukin-10 Overexpression Determines Wound Phenotype and Scar Formation: Modulation of Inflammation and Fetal Healing. Wound Repair Regen. 2014, 22, 406–414.
- Ding, J.; Ma, Z.; Liu, H.; Kwan, P.; Iwashina, T.; Shankowsky, H.A.; Wong, D.; Tredget, E.E. The Therapeutic Potential of a C-X-C Chemokine Receptor Type 4 (CXCR-4) Antagonist on Hypertrophic Scarring in Vivo: CXCR4 Antagonist Minimizes Scar Formation. Wound Repair Regen. 2014, 22, 622–630.
- Dulauroy, S.; Di Carlo, S.E.; Langa, F.; Eberl, G.; Peduto, L. Lineage Tracing and Genetic Ablation of ADAM12+ Perivascular Cells Identify a Major Source of Profibrotic Cells during Acute Tissue Injury. Nat. Med. 2012, 18, 1262–1270.
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.-L.; Gabbiani, G. The Myofibroblast. Am. J. Pathol. 2007, 170, 1807–1816.
- Schmidt, B.A.; Horsley, V. Intradermal Adipocytes Mediate Fibroblast Recruitment during Skin Wound Healing. Development 2013, 140, 1517–1527.
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372.
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human Bone Marrow Stromal Cells Suppress T-Lymphocyte Proliferation Induced by Cellular or Nonspecific Mitogenic Stimuli. Blood 2002, 99, 3838–3843.
- Loots, M.A.M.; Lamme, E.N.; Zeegelaar, J.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Differences in Cellular Infiltrate and Extracellular Matrix of Chronic Diabetic and Venous Ulcers Versus Acute Wounds. J. Investig. Dermatol. 1998, 111, 850–857.
- Nuschke, A. Activity of Mesenchymal Stem Cells in Therapies for Chronic Skin Wound Healing. Organogenesis 2014, 10, 29–37.
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Liu, J.; Dong, L.; Fu, X.; Han, W. Mesenchymal Stem Cell-Conditioned Medium Accelerates Wound Healing with Fewer Scars: Mesenchymal Stem Cell-Conditioned Medium Enhance Wound Scarless Healing. Int. Wound J. 2017, 14, 64–73.
- Cheon, S.S.; Cheah, A.Y.L.; Turley, S.; Nadesan, P.; Poon, R.; Clevers, H.; Alman, B.A. β-Catenin Stabilization Dysregulates Mesenchymal Cell Proliferation, Motility, and Invasiveness and Causes Aggressive Fibromatosis and Hyperplastic Cutaneous Wounds. Proc. Natl. Acad. Sci. USA 2002, 99, 6973–6978.
- Sato, M. Upregulation of the Wnt/beta-Catenin Pathway Induced by Transforming Growth Factor-beta In Hypertrophic Scars and Keloids. Acta Derm. Venereol. 2006, 86, 300–307.
- Lee, W.J.; Park, J.H.; Shin, J.U.; Noh, H.; Lew, D.H.; Yang, W.I.; Yun, C.O.; Lee, K.H.; Lee, J.H. Endothelial-to-Mesenchymal Transition Induced by Wnt 3a in Keloid Pathogenesis: EndoMT in Keloids and Dermal Microvascular Endothelial Cells. Wound Repair Regen. 2015, 23, 435–442.
- Cheon, S.S.; Wei, Q.; Gurung, A.; Youn, A.; Bright, T.; Poon, R.; Whetstone, H.; Guha, A.; Alman, B.A. Beta-catenin Regulates Wound Size and Mediates the Effect of TGF-beta in Cutaneous Healing. FASEB J. 2006, 20, 692–701.
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of Canonical Wnt Signalling Is Required for TGF-β-Mediated Fibrosis. Nat. Commun. 2012, 3, 735.
- Bastakoty, D.; Saraswati, S.; Cates, J.; Lee, E.; Nanney, L.B.; Young, P.P. Inhibition of Wnt/β-catenin Pathway Promotes Regenerative Repair of Cutaneous and Cartilage Injury. FASEB J. 2015, 29, 4881–4892.
- Lee, S.-H.; Kim, M.-Y.; Kim, H.-Y.; Lee, Y.-M.; Kim, H.; Nam, K.A.; Roh, M.R.; Min, D.S.; Chung, K.Y.; Choi, K.-Y. The Dishevelled-Binding Protein CXXC5 Negatively Regulates Cutaneous Wound Healing. J. Exp. Med. 2015, 212, 1061–1080.
- Driskell, R.R.; Watt, F.M. Understanding Fibroblast Heterogeneity in the Skin. Trends Cell Biol. 2015, 25, 92–99.
- Hoffmeyer, K.; Raggioli, A.; Rudloff, S.; Anton, R.; Hierholzer, A.; Del Valle, I.; Hein, K.; Vogt, R.; Kemler, R. Wnt/β Catenin Signaling Regulates Telomerase in Stem Cells and Cancer Cells. Science 2012, 336, 1549–1554.
- Liu, H.; Liu, Q.; Ge, Y.; Zhao, Q.; Zheng, X.; Zhao, Y. HTERT Promotes Cell Adhesion and Migration Independent of Telomerase Activity. Sci. Rep. 2016, 6, 22886.
- Liu, Q.; Sun, Y.; Lv, Y.; Le, Z.; Xin, Y.; Zhang, P.; Liu, Y. TERT Alleviates Irradiation-Induced Late Rectal Injury by Reducing Hypoxia-Induced ROS Levels through the Activation of NF-ΚB and Autophagy. Int. J. Mol. Med. 2016, 38, 785–793.
- Cao, Y.; Xu, L.; Yang, X.; Dong, Y.; Luo, H.; Xing, F.; Ge, Q. The Potential Role of Cycloastragenol in Promoting Diabetic Wound Repair In Vitro. BioMed Res. Int. 2019, 2019, 7023950.
- Zhang, H.; Nie, X.; Shi, X.; Zhao, J.; Chen, Y.; Yao, Q.; Sun, C.; Yang, J. Regulatory Mechanisms of the Wnt/β-Catenin Pathway in Diabetic Cutaneous Ulcers. Front. Pharmacol. 2018, 9, 1114.
- Pellegrini, G.; Ranno, R.; Stracuzzi, G.; Bondanza, S.; Guerra, L.; Zambruno, G.; Micali, G.; De Luca, M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin1. Transplantation 1999, 68, 868–879.
- Walmsley, G.G.; Maan, Z.N.; Wong, V.W.; Duscher, D.; Hu, M.S.; Zielins, E.R.; Wearda, T.; Muhonen, E.; McArdle, A.; Tevlin, R.; et al. Scarless Wound Healing: Chasing the Holy Grail. Plast. Reconstr. Surg. 2015, 135, 907–917.
- De Luca, M.; Pellegrini, G.; Green, H. Regeneration of Squamous Epithelia from Stem Cells of Cultured Grafts. Regen. Med. 2006, 1, 45–57.
- Carsin, H.; Ainaud, P.; Le Bever, H.; Rives, J.-M.; Lakhel, A.; Stephanazzi, J.; Lambert, F.; Perrot, J. Cultured Epithelial Autografts in Extensive Burn Coverage of Severely Traumatized Patients: A Five Year Single-Center Experience with 30 Patients. Burns 2000, 26, 379–387.
- Gallico, G.G.; O’Connor, N.E.; Compton, C.C.; Kehinde, O.; Green, H. Permanent Coverage of Large Burn Wounds with Autologous Cultured Human Epithelium. N. Engl. J. Med. 1984, 311, 448–451.
- Jang, H.; Kim, Y.H.; Kim, M.K.; Lee, K.H.; Jeon, S. Wound-Healing Potential of Cultured Epidermal Sheets Is Unaltered after Lyophilization: A Preclinical Study in Comparison to Cryopreserved CES. BioMed Res. Int. 2013, 2013, 907209.
- Jackson, C.J.; Tønseth, K.A.; Utheim, T.P. Cultured Epidermal Stem Cells in Regenerative Medicine. Stem Cell Res. Ther. 2017, 8, 155.
- Lootens, L.; Brusselaers, N.; Beele, H.; Monstrey, S. Keratinocytes in the Treatment of Severe Burn Injury: An Update. Int. Wound J. 2013, 10, 6–12.
- Wurzer, P.; Keil, H.; Branski, L.K.; Parvizi, D.; Clayton, R.P.; Finnerty, C.C.; Herndon, D.N.; Kamolz, L.P. The Use of Skin Substitutes and Burn Care—A Survey. J. Surg. Res. 2016, 201, 293–298.
- Nicholas, M.N.; Yeung, J. Current Status and Future of Skin Substitutes for Chronic Wound Healing. J. Cutan. Med. Surg. 2017, 21, 23–30.
- Varkey, M.; Ding, J.; Tredget, E.E. Fibrotic Remodeling of Tissue-Engineered Skin with Deep Dermal Fibroblasts Is Reduced by Keratinocytes. Tissue Eng. Part A 2014, 20, 716–727.
 Encyclopedia
Encyclopedia



