Introduction
Food, dairy, maritime, water systems, oil, paper, opticians, dentistry, hospitals, and domestic households are several examples of industries that are affected by the biofilms [1–8]. The occurrence of biofilms leads to serious problems, e.g., product spoilage, reduced production efficiency, corrosion, unsightliness, infection, pipe blockages, and equipment failure [9,10]. A biofilm is defined as an organized aggregate of microorganisms living within an extracellular polymeric matrix that they produce. This is irreversibly attached to either a material surface or a living surface, which cannot be easily removed due to its complex structure [11,12]. This complex layer of extracellular polymeric substances (EPS) protects them from antibiotics, disinfectants, and dynamic environments [13,14]. The intercellular communication within the biofilm rapidly stimulates the up and down-regulation of gene expression. This property enables temporary adaptations, such as phenotypic variation and the ability to survive in nutrient deficient conditions. These pitfalls and challenges create an industrial interest in developing materials, methods, and tools that can prevent or remove the formation of biofilms.
The food and beverage industries operate their production units under stringent hygiene standards to verify high-quality products. However, the presence of biofilms can cause hygienic problems in the case of pathogenic organisms. Biofilms consist of complex microbial communities that adhere to the surfaces fencing processes, like cleaning and disinfection [15]. Biofilms are observed in processing equipment and open surfaces, resulting in food safety problems or a weakening of the efficiency of the production lines [16]. It is important to control the formation of biofilms to avoid corrosion and contamination. This review provides a brief of the biofouling process, including the production mechanisms and control of microbial adhesion.
Bacterial Growth
The growth of a bacterium is defined as the increase in cells number rather than the cell size. There are certain requirements for bacterial growth and factors that influence the growth process. Temperature, pH, osmotic pressure, mixing, and oxygen concentration are the main factors influencing bacterial growth. There are also nutritional requirements, besides these factors. The replication process is not through mitoses and meiosis, but through a process that is known as binary fission. The DNA of a singular bacterium will be replicated during binary fission (Figure 1). Afterwards, the DNA will be divided, and the cell wall and the membrane will form a transverse septum. The transverse septum will be completely formed, which will enable cells separation, thus generating new bacterial cells. The time interval required for the cell division is one pivotal parameter in bacterial growth, which is known as generation time. The generation time depends on the type of organism and the environmental conditions.
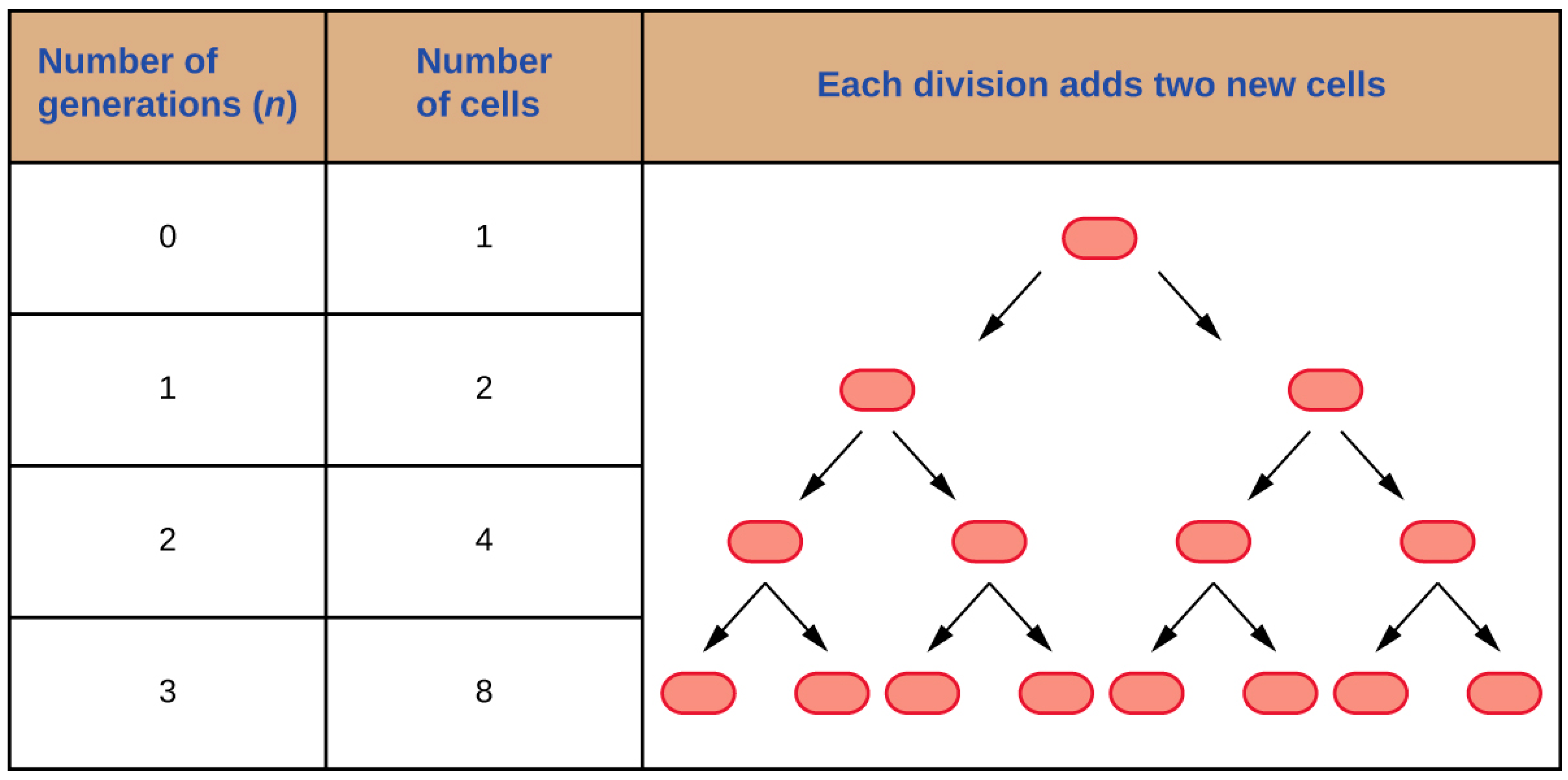
Figure 1. This image displays the binary fission process [17].
The bacterial growth consists of four growth phases, which are depicted in Figure 2. In the first phase, the so-called lag phase, the bacteria must physiologically adapt to the culture conditions and growth begins after a period of time. The density in which the cells are distributed is another important factor. Broadly, exoenzymes and nutrients are shared in proximity; however, if the density is low, this could lead to a dilution of these materials [16]. The latter results in slow cell growth and transition to the log phase. Depending on the conditions, the lag phase may take only minutes or several hours. The time length of the lag phase depends on the type of medium.
The lag phase is followed by the log or exponential phase. In this phase, the cells are in an optimum growth state and they perform binary fission. During the log phase, there is a logarithmic increase in cell number. The slope of this line is the bacterial growth. The mathematical description of the bacterial growth in the log phase can be described while using the following equation:
|
|
δN/δt=μN |
(1) |
where N is defined as the cell number, t as the time and µ as the specific growth rate constant (1/time).
This leads to the following expression for the number of present cells (exponential growth), which is given by:
|
|
N=N0eμt |
(2) |
where N0 is the number of cells at t = 0.
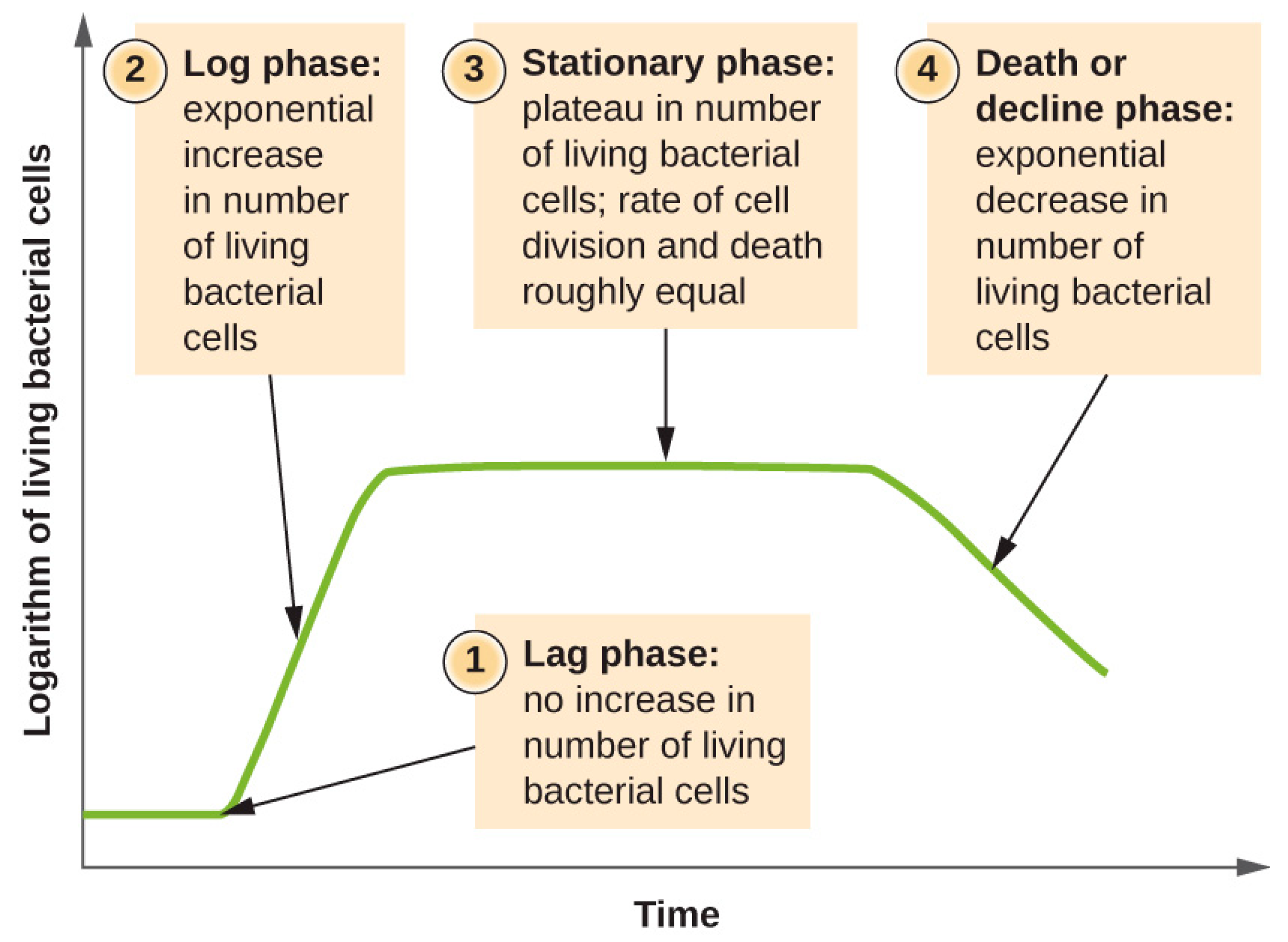
Figure 2. This graph shows the relation between time and the growth of bacteria in a laboratory environment [17].
In the stationary phase, the bacterial growth slows down until it reaches maximum growth and the logarithmic cell number remains constant. The mathematical expression of the stationary phase is expressed as:
|
|
δN/δt=0 |
(3) |
There is a balance between cell division and cell death and there is no actual bacterial growth. This is caused by the depletion of critical nutrients or the accumulation of waste product. Some of the cells can lyse nutrients from the dead cells (endogenous metabolism). This process occurs throughout the whole growth cycle, but occurs in a high degree in the stationary phase. The last phase is called the death or decline phase and accumulation of waste products or exposure to oxygen occurs. The death phase can be mathematically expressed as:
|
|
δN/δt=−kdN |
(4) |
where kd is the specific death rate.
Biofilms
The process of biofilm formation can be summarized in four steps: initial attachment, microcolony formation, maturation, and dispersion (Figure 3). The first step is the process where planktonic bacteria get close to the material surface. This is caused by a combination of physical forces (e.g., attractive van der Waals and repulsive electrostatic forces) and chemical forces (e.g., the cohesion between microbial cells) [6]. The appendages (e.g., fimbriae, pili, and flagella) strengthen the attachment between the bacterial cell and the surface once the bacteria get in touch with the material surface [11]. Moreover, environmental factors, such as temperature and pH, influence the bacterial adhesion. The hydrophobicity of the material surface also reduces certain repulsion forces between the bacteria and material [6]. Di Ciccio et al. alluded that bacteria more likely attach to hydrophobic surfaces (polystyrene) than hydrophilic surfaces (stainless steel) [18]. It is notable that the bacterial attachment is still reversible, which makes it relevant for the prevention of biofilm growth.
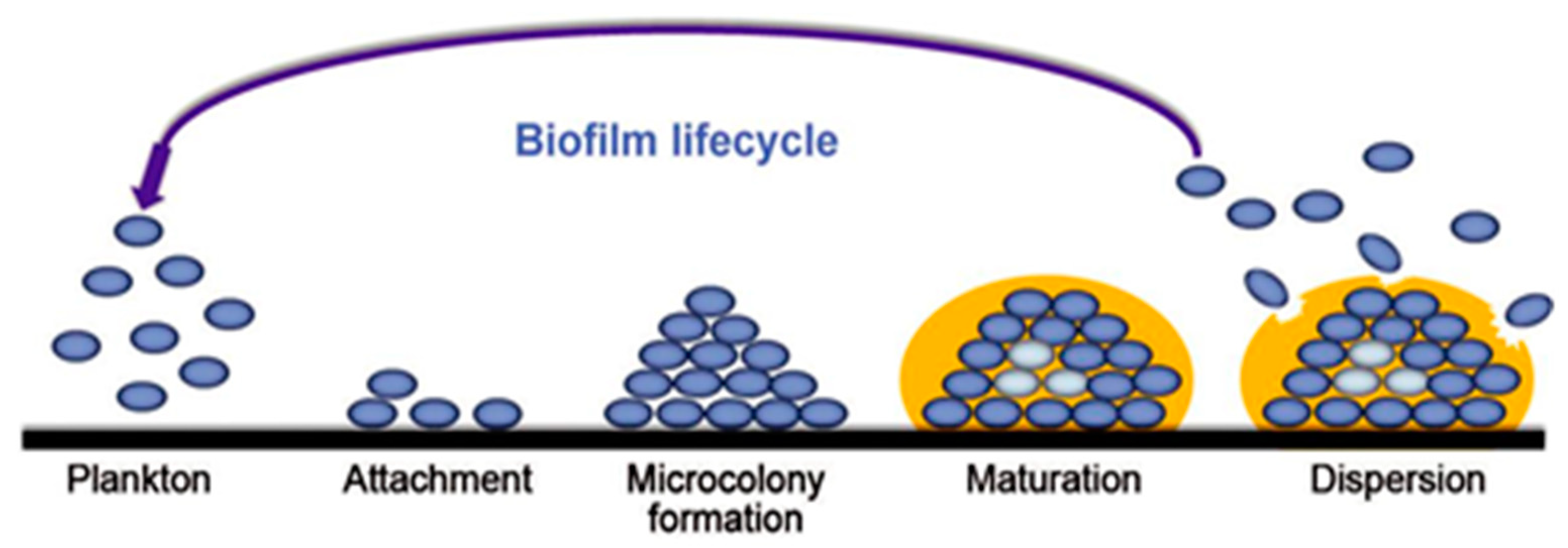
Figure 3. The formation steps of biofilm [19].
The process of multiplication and division of the bacteria starts following the attachment stage. This leads to the EPS originating as a layer that protects the bacteria and this stage is also called irreversible attachment [19,20]. The complex structure of multiple different microcolonies that formed on the surface renders them more resistant to antibiotics and disinfectants. The amount of antibiotic that is needed can range between 100–1000 times more than free-swimming planktonic bacteria [13]. The reciprocal actions between the microcolonies affect the metabolic products [21].
In this stage, certain molecules, the so-called autoinducers, are used as signaling molecules to communicate from cell to cell [22]. The autoinducers make use of a technique called quorum sensing. This communication mechanism between the microbial cells makes the EPS matrix able to adapt to multiple situations. The protection of the complex EPS architecture provides the bacteria the freedom to grow into a mushroom-like structure [23]. Channels are formed between the different forming colonies within the three-dimensional structure of EPS. The channels function as a circulatory water system that allows for nutrients to venture deep into the biofilm and prevent the bacterial growth termination [24]. In the last stage, an oversaturation of microbial cells is observed. Bacteria will disperse from the biofilm into the liquid phase once the EPS temporarily removes the protective layer at the top of the mushroom-like structure. The sessile cells are converted into motile forms, resulting in a dispersion of the microbial cells.
Environmental Factors
Effect of pH
PH plays an intrinsic role in bacterial growth and the optimal range (6.5–7.2) highly depends on the type of bacteria [25]. If the pH deviates from the optimal range may impede the bacterial growth, resulting in bacterial death and the formation of the biofilm stops. However, this is only the case in the first stage of biofilm formation. Bacteria become more acidic resistant after the initial attachment phase, due to the protection from the EPS matrix. While, planktonic bacteria are more acidic sensitive, if the bacteria form a biofilm the bacteria become more resistant to a lower pH [26]. Furthermore, the speed at which the pH of the solution changes affects biofilm development. According to Li et al. [27], a gradual change in the acidity increases the chance of cell survival when compared to a sudden change in pH that was caused by adding HCl. This means that biofilms are less resistant to large pH fluctuations when compared to small pH differences. If this mechanism that lets the biofilm adapt, it is not fast enough due to sudden changes in their environment, and this could lead to cell extermination [10].
Rheological and Adhesive Properties of Biofilms
A previous study investigated the viscoelasticity of biofilms of Pseudomonas aeruginosa and observed dual behavior: an irreversible viscous deformation and viscoelastic response and recoil [28]. The characterization of the biofilm internal structure requires an assessment of physical properties on the micro scale, as well as any structural features that exist within it [29]. However, a recent study by Kundukad et al. has shown that the rheological properties can be independent of the flow rate, a fact that is not documented in earlier observations [30]. They also stated that the size of the microcolonies plays an important role in surface rheology. The reasoning behind this is that the biofilm growth occurs at the periphery, rather than within, the biofilm and shows that the periphery of the microcolonies is compositionally and mechanically dynamic.
Effect of Temperature
The metabolism of bacteria is directly associated with the presence of enzymes, which means that the biofilm formation depends on the reaction rates of the enzymes. A pivotal factor that affects the enzymatic reaction rate is the temperature, as it is correlated to the formation of cells forming a biofilm [10]. Furthermore, studies have shown that the bacteria have an increased surface area when compared to higher temperatures (35 °C) at low temperatures (10 °C) [31–35]. A former study by Townsly and Yildiz examined the temperature influence on the biofilm formation in Vibrio cholerae [36]. They deduced that biofilms derived at 15 °C and 25 °C showed elevated thickness and better structure when compared to those that formed at 37 °C. This is ascribed to the number of appendages. The number of flagella from bacteria is dependent on the temperature.
A bacteria seems to have only a single flagellum and at a lower temperature, the number of flagella increases, and thus increases the surface area of the bacteria and the likelihood of bacterial adhesion in the initial attachment stage at high temperatures (>35 °C) [37]. In spite of the fact that the effect of temperature on the development of biofilms, especially in the first stage of biofilm formation, is extensively examined and reported; the effect of temperature when the biofilm is already established remain ambiguous standpoint [10]. The preceding study refers that high temperatures (80–90 °C) have little impact on the removal of biofilms, as well as shows that the adherent nature of the biofilm to the surface increases, which is known as the baking effects [38].
Microbial Adhesion
Bacterial Adhesion and Bacterial Growth
Bacterial adhesion is one of the challenges in the field of microbial research. Bacterial adhesion has a negative influence on bacterial growth and mobilization. In order to grow, microorganisms require the right conditions and the amount of nutrients. If bacteria adhere to the material surface, they obtain fewer resources, a fact that subdues the bacterial growth. An addable negative effect of bacterial adhesion is the creation of biofilms layer to the material surface. The biofilm layer reinforces the adhesion phenomenon, slowing the bacterial growth and leading to a deficit of the end-product. The bacterial adhesion can be firstly expounded by the physicochemical interactions between the bacteria and the surface, and secondly by the molecular and cellular interactions between the bacteria and the surface.
Theoretical Models
DLVO Theory
One of the theories that were used to describe the reciprocal influences between bacteria and the surface is the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory. This theory is widely applied to delineate interactions between the colloidal particles or a colloidal particle with a surface. As such, the size of bacteria (0.5–2.0 µm) corresponds to the size of colloidal particles; this theory has been used for the interactions between bacteria and the material surface [39].
The balance between the attractive van der Waals forces ∆GvdW and the electrostatic repulsion forces ∆Gdl from the overlap between the electrical double layer of the cell and the substratum have determined the total interactions ∆Gadh of the bacteria with the material surface. The classical DLVO is expressed as:
|
|
ΔGadh(d)=ΔGvdW(d)+ΔGdl(d) |
(5) |
This formula shows that both forces are dependent on the distance between the cell and the substratum. The van der Waals forces become more dominant and the cell will irreversibly adhere to the surface if the bacterium is close to the surface. On the other hand, if the distance between the cell and substratum becomes higher the van der Waals forces drastically decrease and the Coulomb interactions become more dominant, resulting in the cell repelling from the surface [39]. In addition, the ionicity of the cell is a crucial factor for the bacterial adhesion. If the ionic strength is high, the positively and negatively charged particles will attract naturally towards each other. The particles will naturally repel if the ionic strength is low by having the same charge.
However, reversible adherence may occur when the bacterial cell approaches the surface due to its motility or Brownian motion. In this case, the bacterial cell needs to overcome a certain energy barrier. Once the bacterial cell comes close to the surface it can use its appendages, like pili and flagella, to attach to the surface. These appendages can pierce the energy barrier and bacterial adhesion is facilitated [39]. For clarity sake, the DLVO theory describes the probability of an organism to overcome any electrostatic barrier. Current research showed that there is a relation between the ionic strength and bacterial adhesion, which is consistent with the DLVO theory [40].
Thermodynamic Theory
A physicochemical approach has been used to delineate the bacterial attachment to surfaces and to express the van der Waals, electrostatic and dipole, in terms of free energy, and it can be determined by:
|
|
ΔGadh=γsm−γsl−γml |
(6) |
where γsm, γsl, and γml are the solid-microorganism, solid-liquid, and microorganism-liquid free energies, respectively. If free energy is negative, then the adhesion is favored.
On a more general note, the thermodynamic theory always presumes that the bacterial interaction is reversible, which is not the case. According to the thermodynamic approach, the distance between cell and surface is not important and this differs from the conventional DLVO theory. The thermodynamic theory does not explain or predict the behaviors that were observed in bacterial systems. However, it explicates a common observation; namely, the bacteria with hydrophilic properties prefer hydrophilic surfaces to attach to. The same applies to hydrophobic bacteria that prefer hydrophobic surfaces. Silva-Dias et al. insinuated that hydrophobicity has a momentous part in the bacterial adhesion and the agglomeration of bacterial cells [41].
Extended DLVO Theory
The hydrogen bonding can be limned as an electron-donor/electron-acceptor interaction, namely, the Lewis acid-base interaction [42]. This observation led to the development in the extended DLVO theory, where the hydrophobic/hydrophilic as well as the osmotic interactions were included in the model. Nevertheless, the osmotic interactions in bacterial adhesion could be neglected due to their low influence. This led to the following equation:
|
|
ΔGadh=ΔGvdW+ΔGdl+ΔGab |
(7) |
where ∆GvdW is the Lifshitz-van der Waals interaction, ∆Gdl is the electric double layer interaction, and ∆Gab relates to acid-base interactions. With the addition of hydrophobic and repulsive hydration effects, the extended DLVO theory predicts adhesion better than the conventional DLVO theory. Acid-base interaction also plays an important role in lowering the energy barrier. However, only at a small distance (5 nm) from the surface become functional the acid-base interaction [43].
Material Surface Characteristics and Their Impact on Biofouling
Surface Charge
The surface charge is one of the factors that can affect the binding forces between the bacteria and the surface. Most of the bacterial cells are negatively charged and they show better resistance to the bacterial adhesion [2]. However, there are approaches that use positively charged surfaces (I.E. quaternary ammonium-based compounds). This approach aims at the elimination of bacterial cells on contact. A previous study claimed that high electrostatic forces in cell membranes might remove anionic lipids [44]. A disadvantage of this approach is that the dead cells form a layer on the surface, which reduces the charge; thereby, other types of microbial cells can more easily adhere to the surface [45]. Furthermore, there are types of bacteria that can modify the surface charge. Rzhepishevska et al. seconded that Pseudomonas aeruginosa on a negatively charged surface is an example of changing the surface charge to make it more convenient for other bacterial cells to adhere to the surface [46].
Hydrophobicity
The hydrophobicity is a major feature that influences the interactions between bacteria and the surface. Preceding reports contend that superhydrophobic (>150°) and superhydrophilic (0°) surfaces seem to have an impact on bacterial adhesion [47]. According to Huan Zhu et al., the chemical composition, alteration of the surface geometry, as well as the application of external stimulation affect the translation of the superhydrophobic surfaces-based adhesion and its induced transition [48]. Yuan et al. cited that the solid area fraction of a superhydrophobic surface was eliminated due to the entrapped air impeding the adhesion. It is remarkable that surface ability for self-cleaning against bacteria, as well as subsequent applied washing, lead to the reduction of the adhesion [49]. Hizal et al. claimed that the combination of superhydrophobic surfaces and fluid shear stress reduced the adherence of Staphylococcus aureus and Escherichia coli, due to the slipperiness of the superhydrophobic surface [50].
Surface Roughness
An intensified surface roughness can be directly related to increased bacterial adhesion [51]. The surface roughness is related to an increase in surface area, which makes it easier for the bacteria to attach. Furthermore, a rougher surface protects the bacterial cells from fluid shear forces [45,52]. Nevertheless, Renner et al. alleged that there is no such a ‘one size fits all’ relationship between surface roughness and bacterial attachment, owing to the variation of bacterial strains in the size and shape, as well as the effect from the environmental conditions [53].
Surface Topographical Configuration
Recent studies have manifested that the way that peaks and valleys are distributed along the surface also plays an important role in microbial biofilm formation [45,53]. The surface typography around the same diameter of the bacterial cell may entrap bacteria on a stainless steel surface [54]. Parameters, like skewness and kurtosis, need to be considered to understand bacterial cell attachment based on the typography of the surface.
Techniques for the Observation and Measurement of Bacterial Adhesion
There are several different techniques that are used to determine and estimate the bacterial adhesion. Azeredo et al. made an overview of the different techniques used and separated all of the techniques into four groups: chemical, physical, microscopy, and biological [55]. He also mentioned the advantages and limitations of each technique, which are displayed in Figure 4.
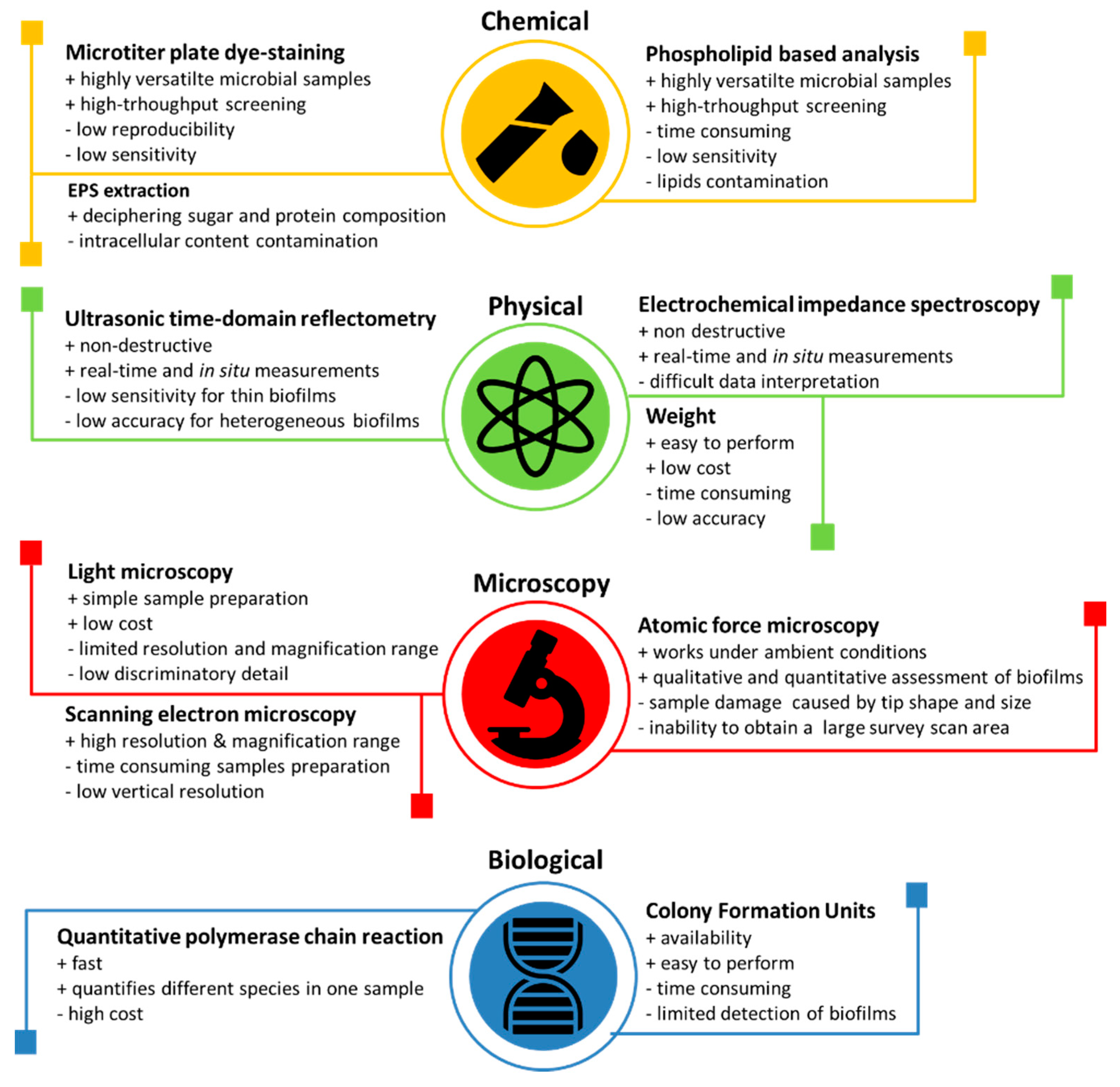
Figure 4. Biofilm detection and measuring techniques [55–58].
Control of Bacterial Adhesion
Microbes adhere to surfaces, enhancing their resistance in antibiotics or the environmental conditions. Research efforts aim to improve the control of adhesion process and several techniques have been investigated and reported [59–63]. This section describes the advantages and limitations of the control techniques.
Antibacterial Agent Release Coatings
Use of antibacterial agents is a successive and simple option to biofilms. Microbes in a biofilm are more protected due to the adaptability of the EPS layer protecting the bacteria from antibiotics [64]. Biofilms-based pathogenic bacteria resist antibiotics and this fact is ascribed to long-standing bacterial infections. Essentially, a release-based coating exerts antibacterial activity by accoutering antibacterial compounds over time. The antibacterial compounds make sure that both adherent and adjacent planktonic bacteria are killed during the process. The purvey of antibacterial agents is attained by diffusion into the aqueous medium [44]. The advantage of antibacterial agents is that the elution from the material surface locally increases the concentration of the antibacterial agent, without transcending the toxicity limits, when compared to traditional delivery methods for antibiotics. It accouters antibacterial substances only where there is need of, thus eliminating resistance activity and obviating the potentially deleterious systemic incidences. Several design strategies that are used with antibacterial agent release coatings are described in a review that was conducted by Cloutier et al. [65] These strategies can be separated into three groups: passive approaches, active approaches, and bacteria triggered approaches [65].
The passive approach focuses on the different variables that have shown to be an influence on release kinetics and that do not have an active trigger when to release antibacterial agents. This can be based on either the properties of the antibacterial agent, the carrier matrix, or the overall micro/nanostructure of the antibacterial coating [66,67]. On the other hand, the active approach uses stimuli-responsive materials. These types of coatings require a trigger before releasing the antibacterial agents. Physical exogenous stimuli are the nost commonly used triggers. According to Cloutier et al., these approaches offer great signal control and they are not restricted by diffusion, which gives them the potential for the use of bioactive coatings [65]. These bioactive coatings can produce antibacterial agents on demand. This also leads to improved useful longevity of the coating [68].
Lastly, bacteria-triggered approaches activate the release profiles of antibacterial agents when the bacteria are in the vicinity or are getting in touch with the coating. There are two ways of triggering the release of antibacterial agents: (1) pH-responsive coatings and (2) enzyme responsive coatings. In the process of bacterial growth, metabolism occurs where acidic acids, like lactic acid or acetic acids, are produced. Zhuk et al. used poly-electrolyte multilayers (PEM) films as a release mechanism [69]. The antibiotics release activity is influenced from the pH conditions. Lu et al. used self-defensive layer-by-layer coatings in the form of hydrogels stimulating the antibiotic release [70]. It is also cited that another mechanism makes use of the cleavage of imine bonds [71].
The enzyme responsive coatings have an advantage in comparison to the pH-responsive coatings. Enzyme-responsive coatings target specific bacteria, because they can differentiate between the different bacteria strains. However, this approach is new and is not well known. Up to now, two distinct ways of release have been reported, which are coating degradation and the cleavage of chemical bonds [72–74]. Coatings with multiple functions are necessary to achieve better performance in their environments and for specific applications due to the complexity and the hierarchical structure of the biological systems, and they are divided into three groups: multi-release, multi-approach, or multi-property [65].
Contact Killing Coatings
Immobilizing antibacterial biocides onto the surface platform by covalent bonding creates a contact killing surface (or so-called contact active surface). However, contact killing surfaces do not have any bacterial adhesive resistance, instead of eliminating bacteria that encounter the surface. This is facilitated by either physical damage or by nonoxidative stress [75]. There are multiple forms of contact killing coatings that are defined by the type of antibacterial agent attached to the surface and the proposed mechanisms of action in which the bacteria are eliminated. The most commonly reported as contact active antibacterial agents include quaternary ammonium compounds (QACs), such as alkyl pyridiniums and quaternary poly (2-(dimethylamino) ethyl methacrylate), quaternary phosphonium (QPs), and N-chloramines [75]. Green reports that QACs are often used as an antimicrobial agent (AMA) for contact killing coatings and it denotes the distinction between QACs based on silanes (Si-QAC) and QACs based on polymers [76]. Furthermore, a wide range of molecules has been tested as AMA, including guidelines, enzymes, chitosan, peptides, and other peptidomimetics [76].
Tiller et al. examined the first bacterial elimination mechanism [77]. According to them, the long polymeric strings that were attached to the microbial agents enable penetrating a hole in the membrane of a gram-positive bacterium and with this eventually kill the cell. However, recent studies cite that the length of the polymer chain does not influence the antibacterial impact of the coating. Kugler et al. proposed the second mechanism [78]. According to them, the exchange of ions is responsible for the bacterial killing due to the destabilization and the loss of natural ions of bacteria.
Bieser and Tiller et al. proposed the most recent mechanism, which is called the phospholipid sponge effect [79]. They state that the antimicrobial action occurs by the selective adhesion of negatively charged phospholipids in the bacterial cell membrane onto the cationic surface. A recent study that was performed by Gao et al. confirmed the existence of the phospho-lipid sponge effect [80]. They reported a negative connection between the film cross-linking density and negatively charged phospholipid, whereas the neutral phospholipid exhibited no correlation supporting the action of anionic phospholipid suction that was proposed in the sponge effect. The three mechanisms described above are all based on contact killing QACs coatings, immobilizing either silanes, or polymers as antibacterial agents. The last mechanism is only based on N-chloramines coatings. This mechanism is believed to be revolved around the active chlorine transfer. Nevertheless, further examination of the antibacterial mechanisms of N-chloramine modified surfaces is required [81].
Anti-Adhesion/ Bacteria Repelling Coatings
The coatings aim to prevent the first step in biofilm formation using non-cytotoxic mechanisms, in contrast to the other two forms of antibacterial coatings. Bacterial attachment to the surface occurs through several mechanisms and, if not prevented in the first stage, biofilm formation can lead to serious infections. The control over surface hydrophobicity, charge, roughness, and topographical configuration can drastically reduce the attachment of bacteria.
Poly Ethylene Glycol (PEG) is the most commonly used anti-adhesion coating. PEG chains are pliant and they display steric aversion forces, which hinder the bacteria attachment in the surface [82]. PEG was first used for antifouling coatings against protein absorption, and due to its preventative properties was applied for preventing bacterial adhesion. Ostuni et al. investigated whether the protein and bacteria resistance correlates to an extension to each other [833]. He observed that there was no correlation between the two, and therefore presumed that other parameters are required to prevent the attachment of bacteria, due to the complexity of the mechanisms that result in bacterial attachment. Furthermore, the PEG was not sustainable for long term usage [8484].
Other anti-adhesion coatings make use of surfactants to prevent bacterial attachment. Hydroxyapatite and chitosan or biological molecules are examples of this surfactant, such as albumin and heparin [855]. In a recent study that was performed by Buzzacchera et al., polymer brush functionalized chitosan was used as an antifouling implant coating to prevent bacterial adhesion and diminishing the platelet activation and leukocyte adhesion [86]. The coating was hydrophilic and it reached a thickness of up to 180 nm within 30 min. of polymerization. Another approach for anti-adhesion coatings is based on the hydrophobicity where the superhydrophobic or hydrophilic coatings aggravate the attachment for bacteria to the surface. A more recent study uses a repellent film that is based on flagellin [87]. In this study, a novel type of bacteria repellent layer that was made of the flagellin is examined. This concept showed that the flagellin protein forms an oriented and dense layer on a hydrophobic surface, which resulted in an effective bacterial cell repellent feature. The cost-effectiveness is the big advantage of fabricating flagellin based coatings, because the protein can be rather easily produced in large amounts.
Multifunctional Coatings
Multifunctional coatings are essential for enhanced anti-adhesion performance in a complex and well-structured biological system. They can be divided into three groups: multi-release, multi-approach, or multi-property [65]. The multi-release coatings combine different release mechanisms together, for example, can both have material responsive and bacterial responsive release triggers of antibacterial agents. Multi-approach coatings combine different approaches i.e., a combination of released antibacterial agents, contact-killing, and anti-adhesion coatings [888]. However, for the future prospect in the microbiology industry coatings should focus on multi-property coatings. According to Variola et al., the properties of the surfaces of materials can be modified on a range of scales by various techniques in multifunctional coatings [81]. In this case, the coating should not only prevent bacterial adhesion, but at the same time should have increased wear resistance, corrosion resistance, or anticoagulation [89].
References
- Dang, H.; Lovell, C.R. Microbial surface colonization and biofilm development in marine environments. Mol. Biol. Rev. 2016, 80, 91–138.
- Ashbolt, N.J. Microbial contamination of drinking water and human health from community water systems. Environ. Health Rep. 2015, 2, 95–106.
- Neyret, C.; Herry, J.M.; Meylheuc, T.; Dubois-Brissonnet, F. Plant-derived compounds as natural antimicrobials to control paper mill biofilms. Ind. Microbiol. Biotechnol. 2014, 41, 87–96.
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The human microbiota in health and disease. Engineering 2017, 3, 71–82.
- Vuitton, D.A.; Dalphin, J.C. From farming to engineering: The microbiota and allergic diseases. Engineering 2017, 3, 98–109.
- Bispo, P.J.; Haas, W.; Gilmore, M.S. Biofilms in infections of the eye. Pathogens 2015, 4, 111–136.
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. Clin. Periodontol. 2017, 44, S12–S22.
- Moen, B.; Røssvoll, E.; Måge, I.; Møretrø, T.; Langsrud, S. Microbiota formed on attached stainless steel coupons correlates with the natural biofilm of the sink surface in domestic kitchens. J. Microbiol. 2015, 62, 148–160.
- Vigneron, A.; Alsop, E.B.; Chambers, B.; Lomans, B.P.; Head, I.M.; Tsesmetzis, N. Complementary microorganisms in highly corrosive biofilms from an offshore oil production facility. Environ. Microbiol. 2016, 82, 2545–2554.
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Nat. Sci. USA 2008, 18, 1049–1056.
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. Chin. Med. Assoc. 2017, 81, 7–11.
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical regulation of cell behavior—Cross talk between substrate stiffness and nanotopography. Engineering 2017, 3, 36–54.
- Olsen, I. Biofilm-specific antibiotic tolerance and resistance. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886.
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Microbiol. 2015, 6, 705.
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New weapons to ht old enemies: Novel strategies for the (bio) control of bacterial biofilms in the food industry. Microbiol. 2016, 7, 1641.
- Maier, R.M.; Pepper, I.L. Bacterial Growth, in Environmental Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–56.
- C Microbiology, Temparature, and Microbial Growth. OpenStax College Microbiology. 2016. Available online: https://cnx.org/contents/Us0vmjzQ@2.8:UortlRj8@4/Temperature-and-Microbial-Growth (accessed on 19 February 2019).
- Di Ciccio, P.; Vergara, A.; Festino, A.R.; Paludi, D.; Zanardi, E.; Ghidini, S.; Ianieri, A. Biofilm formation by Staphylococcus aureus on food contact surfaces: Relationship with temperature and cell surface hydrophobicity. Food Control 2015, 50, 930–936.
- Dos Santos, A.L.S.; Galdino, A.C.M.; De Mello, T.P.; Ramos, L.D.S.; Branquinha, M.H.; Bolognese, A.M.; Columbano, J.; Roudbary, M.; Neto, J.C. What are the advantages of living in a community? A microbial biofilm perspective! Memórias do Instituto Oswaldo Cruz 2018, 113, doi:10.1590/0074-02760180212.
- Hoffman, M.D.; Zucker, L.I.; Brown, P.J.; Kysela, D.T.; Brun, Y.V.; Jacobson, S.C. Timescales and frequencies of reversible and irreversible adhesion events of single bacterial cells. Chem. 2015, 87, 12032–12039.
- Katsikogianni, M.; Missirlis, Y. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Cells Mater. 2004, 8, 37–57.
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Rev. Cell Dev. Biol. 2005, 21, 319–346.
- Kolter, R.; Greenberg, E.P. Microbial sciences: The superficial life of microbes. Nature 2006, 441, 300.
- Persat, A.; Nadell, C.D.; Kim, M.K.; Ingremeau, F.; Siryaporn, A.; Drescher, K.; Wingreen, N.S.; Baaler, B.L.; Gitai, Z.; Stone, H.A. The mechanical world of bacteria. Cell 2015, 16, 988–997.
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Wound Care 2015, 4, 431–439.
- Liu, Y.; Tang, H.; Lin, Z.; Xu, P. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Adv. 2015, 33, 1484–1492.
- Li, Y.H.; Hanna, M.N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Cell density modulates acid adaptation in Streptococcus mutans: Implications for survival in biofilms. Bacteriol. 2001, 183, 6875–6884.
- Klapper, I.; Rupp, C.J.; Cargo, R.; Purvedorj, B.; Stoodley, P. Viscoelastic fluid description of bacterial biofilm material properties. Bioeng. 2002, 80, 289–296.
- Billings, N.; Birjiniuk, A.; Samad, T.S.; Doyle, P.S.; Ribbeck, K. Material properties of biofilms—A review of methods for understanding permeability and mechanics. Prog. Phys. 2015, 78, 036601.
- Kundukad, B.; Seviour, T.; Liang, Y.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanical properties of the superficial biofilm layer determine the architecture of biofilms. Soft Matter 2016, 12, 5718–5726.
- Nisbet, B.A.; Sutherland, I.W.; Bradshaw, I.J.; Kerr, M.; Morris, E.R.; Shepperson, W.A. XM-6: A new gel-forming bacterial polysaccharide. Polym. 1984, 4, 377–394.
- Herald, P.J.; Zottola, E.A.. Attachment of Listeria monocytogenes to stainless steel surfaces at various temperatures and pH values. Food Sci. 1988, 53, 1549–1562.
- Rampadarath, S.; Bandhoa, K.; Puchooa, D.; Jeewon, R.; Bal, S. Early bacterial biofilm colonizers in the coastal waters of Mauritius. J. Biotechnol. 2017, 29, 13–21.
- Vásquez-Ponce, F.; Higuera-Llantén, S.; Soledad Pavlov, M.; Ramírez-Orellana, R.; Marshall, S.H.; Olivares-Pacheco, J. Alginate overproduction and biofilm formation by psychrotolerant Pseudomonas mandelii depend on temperature in Antarctic marine sediments. J. Biotechnol. 2017, 28, 27–34.
- Govaert, M.; Smet, C.; Baka, M.; Ećimović, B.; Walsh, J.L.; Van Impe, J. Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as Function of Biofilm Age. Sci. 2018, 8, 2702.
- Townsley, L.; Yildiz, F.H. Temperature affects c-di-GMP signalling and biofilm formation in Vibrio cholerae. Microbiol. 2015, 17, 4290–4305.
- Nguyen, H.; Yang, Y.; Yuk, H. Biofilm formation of salmonella typhimurium on stainless steel and acrylic surfaces as affected by temperature and ph level. LWT Food Sci. Technol. 2014, 55, 383–388.
- Marion-Ferey, K.; Pasmore, M.; Stoodley, P.; Wilson, S.; Husson, G.P.; Costerton, J.W. Biofilm removal from silicone tubing: An assessment of the efficacy of dialysis machine decontamination procedures using an in vitro model. Hosp. Infect. 2003, 53, 64–71.
- Hori, K.; Matsumoto, S. Bacterial adhesion: From mechanism to control. Eng. J. 2010, 48, 424–434.
- Fletcher, M.; Savage, D.C. Bacterial Adhesion: Mechanisms and Physiological Significance; Springer Science & Business Media: Berlin, Germany, 2013.
- Silva-Dias, A.; Miranda, I.M.; Branco, J.; Monteiro-Soares, M.; Pina-Vaz, C.; Rodrigues, A.G. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: Relationship among Candida spp. Microbiol. 2015, 6, 205.
- Van Oss, C.J. The forces involved in bioadhesion to flat surfaces and particles—Their determination and relative roles. Biofouling 1991, 4, 25–35.
- Bos, R.; Van der Mei, H.C.; Busscher, H.J. Physico-chemistry of initial microbial adhesive interactions–its mechanisms and methods for study. FEMS Microbiol. Rev. 1999, 23, 179–230.
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554.
- Song, F.; Koo, H.; Ren, D. Effects of material properties on bacterial adhesion and biofilm formation. Dent. Res. 2015, 94, 1027–1034.
- Rzhepishevsk, O.; Hakobyan, S.; Ruhal, R.; Gautrot, J.; Barbero, D.; Ramstedt, M. The surface charge of anti-bacterial coatings alters motility and biofilm architecture. Sci. 2013, 1, 589–602.
- Berne, C.; Ellison, C.K.; Ducret, A.; Brun, Y.V. Bacterial adhesion at the single-cell level. Rev. Microbiol. 2018, 16, 616–627.
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Commun. 2014, 50, 3900–3913.
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261.
- Hizal, F.; Rungraeng, N.; Lee, J.; Jun, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.H. Nanoengineered superhydrophobic surfaces of aluminum with extremely low bacterial adhesivity. ACS Appl. Mater. Interfaces 2017, 9, 2118–2129.
- Dantas, L.C.D.M.; Silva-Neto, J.P.D.; Dantas, T.S.; Naves, L.Z.; das Neves, F.D.; da Mota, A.S. Bacterial adhesion and surface roughness for different clinical techniques for acrylic polymethyl methacrylate. J. Dent. 2016, 2016, doi:10.1155/2016/8685796.
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topo-graphical factors influencing bacterial attachment. Advances in Colloid and Interface Science 2012, 179, 142–149.
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355.
- Palmer, J.; Flint, S.; Brooks, J. Bacterial cell attachment, the beginning of a biofilm. Ind. Microbiol. Biotechnol. 2007, 34, 577–588.
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Mickaël, D.; Bonaventura, G.D.; Hebruad, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Rev. Microbiol. 2017, 43, 313–351.
- Hu, G.; Guan, K.; Lu, L.; Zhang, J.; Lu, N.; Guan, Y. Engineered functional surfaces by laser microprocessing for biomedical applications. Engineering 2018, 4, 822–830.
- Valdebenito-Rolack, E.; Ruiz-Tagle, N.; Abarzúa, L.; Aroca, G.; Urrutia, H. Characterization of a hyperthermophilic sulphur-oxidizing biofilm produced by archaea isolated from a hot spring. J. Biotechnol. 2017, 25, 58–63.
- Anderson, M.S. The Detection of Long-Chain Bio-Markers Using Atomic Force Microscopy. Sci. 2019, 9, 1280.
- Gupta, T.T.; Karki, S.B.; Fournier, R.; Ayan, H. Mathematical Modelling of the Effects of Plasma Treatment on the Diffusivity of Biofilm. Sci. 2018, 8, 1729.
- Satpute, S.K.; Mone, N.S.; Das, P.; Banpurkar, A.G.; Banat, I.M. Lactobacillus acidophilus Derived Biosurfactant as a Biofilm Inhibitor: A Promising Investigation Using Microfluidic Approach. Sci. 2018, 8, 1555.
- Antolak, H.; Mizerska, U.; Berłowska, J.; Otlewska, A.; Kręgiel, D. Quillaja saponaria Saponins with Potential to Enhance the Effectiveness of Disinfection Processes in the Beverage Industry. Sci. 2018, 8, 368.
- Chang, S.; Chen, J.; Shi, L. Using Thermal Shock to Inhibit Biofilm Formation in the Treated Sewage Source Heat Pump Systems. Sci. 2017, 7, 343.
- Bertoglio, F.; Bloise, N.; Oriano, M.; Petrini, P.; Sprio, S.; Imbriani, M.; Tampieri, A.; Visai, L. Treatment of Biofilm Communities: An Update on New Tools from the Nanosized World. Sci. 2018, 8, 845.
- Infante, C.D.; Castillo, F.; Pérez, V.; Riquelme, C. Inhibition of Nitzschia ovalis biofilm settlement by a bacterial bioactive compound through alteration of EPS and epiphytic bacteria. J. Biotechnol. 2018, 33, 1–10.
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial coatings: Challenges, perspectives, and opportunities. Trends Biotechnol. 2015, 33, 637–652.
- Qin, S.; Xu, K.; Nie, B.; Ji, F.; Zhang, H. Approaches based on passive and active antibacterial coating on titanium to achieve antibacterial activity. Biomed. Mater. Res. Part A 2018, 106, 2531–2539.
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. Orthop. Surg. Res. 2015, 10, 157.
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Soc. Rev. 2006, 35, 780–789.
- Zhuk, I.; Jariwala, F.; Attygalle, A.B.; Wu, Y.; Libera, M.R.; Sukhishvili, S.A. Self-defensive layer-by-layer films with bacteria-triggered antibiotic release. ACS Nano 2014, 8, 7733–7745.
- Lu, Y.; Wu, Y.; Liang, J.; Libera, M.R.; Sukhishvili, S.A. Self-defensive antibacterial layer-by-layer hydrogel coatings with pH-triggered hydrophobicity. Biomaterials 2015, 45, 64–71.
- Neqal, M.; Fernandez, J.; Coma, V.; Gauthier, M.; Héroguez, V. Ph-triggered release of an antifungal agent from polyglycidol-based nanoparticles against fuel fungus h. resinae. Colloid Interface Sci. 2018, 526, 135–144.
- Komnatnyy, V.V.; Chiang, W.C.; Tolker-Nielsen, T.; Givskov, M.; Nielsen, T.E. Bacteria-triggered release of antimicrobial agents. Chem. Int. Ed. 2014, 53, 439–441.
- Cado, G.; Aslam, R.; Séon, L.; Garnier, T.; Fabre, R.; Parat, A.; Chassepot, A.; Voegel, J.-C.; Senger, B.; Schneider, F.; et al. Self-Defensive Biomaterial Coating Against Bacteria and Yeasts: Polysaccharide Multilayer Film with Embedded Antimicrobial Peptide. Funct. Mater. 2013, 23, 4801–4809.
- Song, N.; Yang, Y.W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Soc. Rev. 2015, 44, 3474–3504.
- Kaur, R.; Liu, S. Antibacterial surface design–contact kill. Surf. Sci. 2016, 91, 136–153.
- Green, J.B.D.; Fulghum, T.; Nordhaus, M.A. A review of immobilized antimicrobial agents and methods for testing. Biointerphases 2011, 6, MR13–MR28.
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Natl. Acad. Sci. USA 2001, 98, 5981–5985.
- Kügler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348.
- Bieser, A.M.; Tiller, J.C. Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Biosci. 2011, 11, 526–534.
- Gao, J.; White, E.M.; Liu, Q.; Locklin, J. Evidence for the phospholipid sponge effect as the biocidal mechanism in surface-bound polyquaternary ammonium coatings with variable cross-linking density. ACS Appl. Mater. Interfaces 2017, 9, 7745–7751.
- Variola, F.; Vetrone, F.; Richert, L.; Jedrzejowski, P.; Yi, J.-H.; Zalzal, S.; Clair, S.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Improving biocompatibility of implantable metals by nanoscale modification of surfaces: An overview of strategies, fabrication methods, and challenges. Small 2009, 5, 996–1006.
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Mater. 2011, 23, 690–718.
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A survey of structure- property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620.
- Kane, R.S.; Deschatelets, P.; Whitesides, G.M. Kosmotropes form the basis of protein-resistant surfaces. Langmuir 2003, 19, 2388–2391.
- Junter, G.A.; Thébault, P.; Lebrun, L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016, 30, 13–25.
- Buzzacchera, I.; Vorobii, M.; Kostina, N.Y.; de los Santos Pereira, A.; Riedel, T.; Bruns, M.; Ogieglo, W.; Möller, M.; Wilson, C.J.; Rodriguez-Emmenegger, C. Polymer Brush-Functionalized Chitosan Hydrogels as Antifouling Implant Coatings. Biomacromolecules 2017, 18, 1983–1992.
- Kovacs, B.; Patko, D.; Klein, A.; Kakasi, B.; Saftics, A.; Kurunczi, S.; Vonderviszt, F.; Horvath, R. Bacteria repellent layer made of flagellin. Actuators B Chem. 2018, 257, 839–845.
- Yan, S.; Yuan, S.; Yin, J.; Luan, S.; Shi, H.; Xu, X.; Zhang, J.; Yang, Y. Hierarchical polymer brushes with dominant antibacterial mechanisms switching from bactericidal to bacteria repellent. Biomacromolecules 2016, 17, 1696–1704.
- Zheludkevich, M.; Tedim, J.; Ferreira, M. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Acta 2012, 82, 314–323.
