The engineering applications of hydrogels are generally limited by the common problem of their softness and brittleness. In this study, a composite double network ionic hydrogel (CDN-gel) was obtained by the facile visible light triggered polymerization of acrylic acid (AA), polyvinyl alcohol (PVA), and hydrolyzed triethoxyvinylsilane (TEVS) and subsequent salt impregnation. The resulting CDN-gels exhibited high toughness, recovery ability, and notch-insensitivity. The tensile strength, fracture elongation, Young’s modulus, and toughness of the CDN-gels reached up to ~21 MPa, ~700%, ~3.5 MPa, and ~48 M/m3, respectively. The residual strain was below 25% after stretch-releasing of 1000 cycles at a strain of 200%. These properties will enable greater application of these hydrogel materials, especially for the fatigue resistance of tough hydrogels, as well as broaden their applications in damping.
- double network ionic hydrogel
- multi-sacrifice bonds
- high toughness
- fast recovery
- notch-insensitivity
Visible light is a safe, low cost, and easily acquired trigger that has attracted attention in synthesis of polymers via radical-initiated polymerization in recent years [22,23]. Figure 1 presents the preparation process and interactions of the CDN-gel. The alkoxy groups are first hydrolyzed into silanol groups in the presence of water, and the silanol groups then condensate to form a siloxane bond [24,25]. The free-radical grafting polymerization mechanism of the PVA/PAA hydrogel triggered by visible light with a CQ initiator was proposed in our previous work [20]. Specifically, CQ uses light to dissociate the initiator molecules into free radicals, which react with double bonds in the monomers or pre-polymers, thus allowing crosslinking to occur. In this work, monomer AA not only grafted with VSTO, leading to the formation of nanobrush gelators, but was also polymerized on the PVA backbone, resulting in a polymer network with a grafted structure. Simultaneously, the silanol can be adsorbed to the PVA on its OH-rich chains through hydrogen bonding, further leading to siloxane bridges [25]. Finally, the visible light-induced hydrogels were exposed to a saturation lithium solution to increase the crosslinking sites between the polymer chains by metal doping. The CDN-gels created by this method were expected to have a network structure constructed through a combination of ion-mediated reversible physical cross-linking, intra- and inter-polymer chain hydrogen bonding, physical entanglement of the polymer chains, and covalent cross-linking.
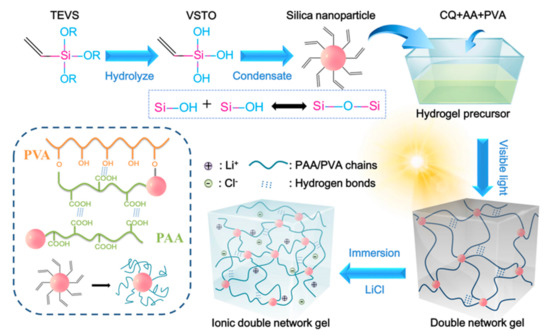
Figure 1. Schematic diagram of the preparation process and network structure of the composite double network ionic hydrogel (CDN-gel). PVA, polyviny alcohol; PAA, poly (acrylic acid); TEVS, triethoxyvinylsilane; VSTO, vinyl silanetriol; AA, acrylic acid; CQ, camphorquinone.
This entry is adapted from the peer-reviewed paper 10.3390/polym12102263
 Encyclopedia
Encyclopedia


