The exploitation of solar energy, an unlimited and renewable energy resource, is of prime interest to support the replacement of fossil fuels by renewable energy alternatives. Solar energy can be used via concentrated solar power (CSP) combined with thermochemical energy storage (TCES) for the conversion and storage of concentrated solar energy via reversible solid–gas reactions, thus enabling round the clock operation and continuous production. This entry is about High-temperature thermochemical energy storage using the enthalpy of reversible reactions.
- thermochemical energy storage
- solid-gas reaction
- hydroxide
- concentrated solar power
- reversible reactions
1. Introduction
The enthalpy of solid-gas chemical reactions stored in chemical materials can be used to generate heat when necessary via endothermal/exothermal reversible reactions. The stored and released heat can be used for example to run power cycles or more generally in industrial processes operating at high temperatures and thus requiring high amounts of energy that are usually provided by fossil fuel combustion. Thus, thermochemical energy storage (TCES) has potential to lower fossil fuel consumption and related greenhouse gas emissions [1]. A high potential also exists in the combination of TCES systems with renewable energy systems. Thermal energy storage is indeed particularly suitable for being combined with concentrated solar energy that relies on an intermittent resource, with the aim to operate the process continuously (day and night as well as stable operation during fluctuating solar energy input) (Figure 1). Indeed, solar energy is variable and can fluctuate a lot in nature due to clouds and weather conditions, thus requiring a storage system for smooth and stable operation under fluctuating solar irradiation conditions. TCES is thus attractive since continuous operation allows a strong increase in the capacity factor of the solar plant, while it can further contribute to eliminating transient effects due to start-up/shutdown periods and unstable/variable solar conditions. The possible envisioned applications are pertaining to electricity production by concentrated solar power (CSP) plants or more generally high temperature chemical processes requiring an external energy input as the process heat supply (e.g., cement and concrete production, minerals calcination, metallurgical processes, fuel production processes or chemical industrial processes). Most industrial energy-intensive processes require a high temperature heat source generally provided by fossil fuel burning. In such high temperature processes, the required high temperature heat for running power cycles or driving endothermal reactions can be generated with solar concentrating systems (parabolic dish, trough, linear Fresnel systems or solar tower receivers with heliostat field). This is the case of CSP plants for electricity generation and solar thermochemical processes for fuels (syngas production via reforming, gasification of carbonaceous feedstocks, H2O and CO2 splitting via thermochemical cycles, etc.) or chemical commodity production (cement, metals, etc.). Thus, the interest in TCES integration in such processes for continuous operation is constantly growing. Another possible application is the utilization of TCES for the recovery and storage of waste heat of various energy and industrial processes at different temperature levels in order to increase process efficiencies or to produce additional extra heat/electricity.
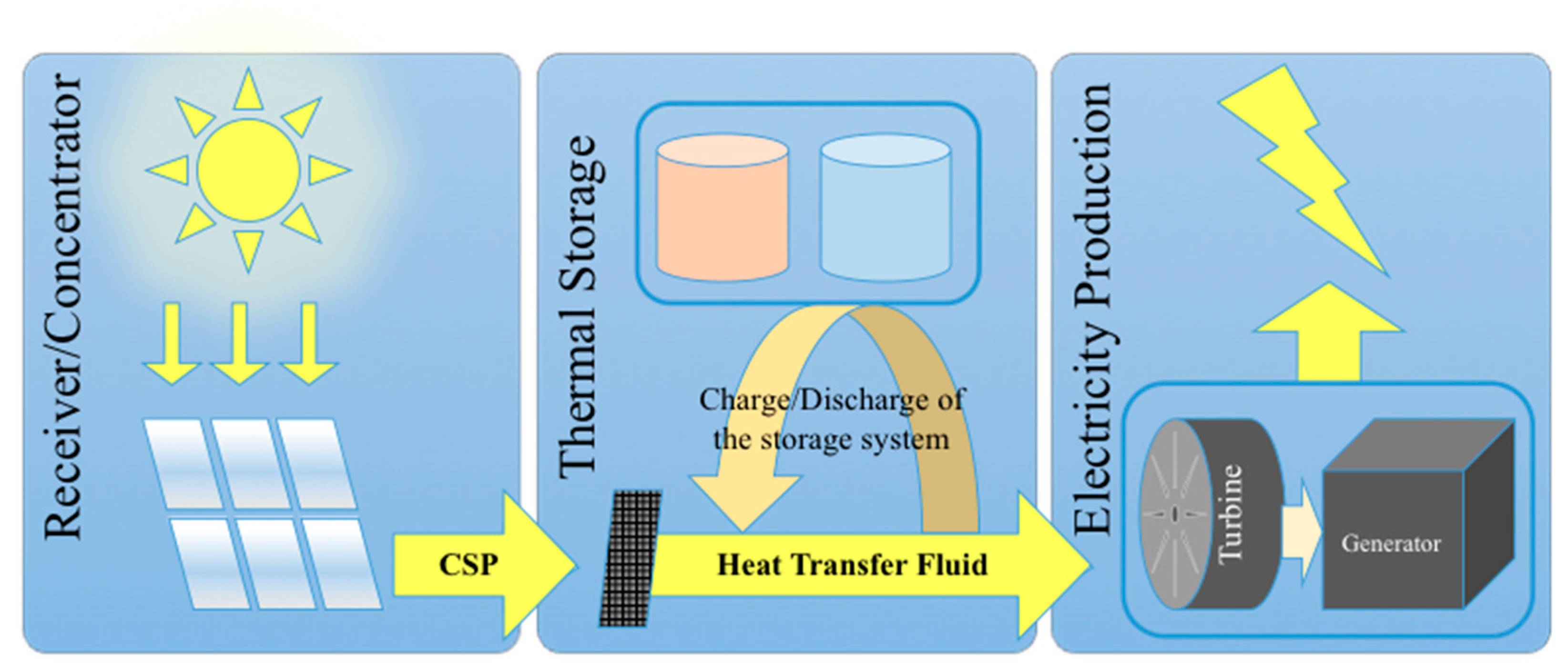
Figure 1. Scheme of the solar power plant main components integrating buffer thermal energy storage system.
In contrast to other energy storage systems including sensible and/or latent energy storage, thermochemical storage offers the possibility of high energy densities in the form of chemical bonds as well as long-term storage and long-range transport in the form of stable and safe materials (Table 1). In addition, the operating conditions can be tuned in a wide range of temperatures and pressures depending on the used TCES system and involved chemical reactions, thus offering the possibility of being combined with various processes. In contrast to sensible or latent heat storage systems that have been developed and optimized, and are even commercially available and applied at large scale, thermochemical energy storage is a new research area in which many aspects are still unknown and are still to be discovered [2]. Research advances are thus needed for potential industrial implementation, while also taking into account the energy consumption by auxiliary equipment and feedstock cost that impact the system capital cost [3]. The main fields in which strong efforts are necessary to develop practical TCES systems and bridge the gap from fundamental research to application are the discovery of cost effective, abundant and affordable chemical materials with high energy densities, cycle stability and fast kinetics for heat storage and release [1]. Furthermore, additional research and technological developments are needed in the optimal design of heat storage-chemical reactor systems for maximum heat transfer between the storage medium and the high temperature solar process, and the complete system integration in large scale plants (optimization of heat and mass flows, dynamic simulation during transient events and fluctuating solar conditions, techno-economics, etc.) [4][5].
Table 1. Comparison of the main options for thermal energy storage using concentrated solar power (CSP), adapted with permission from [6][7], Elsevier, 2020.
|
Storage Type |
Sensible Heat Storage (SHS) |
Latent Heat Storage (LHT) |
Thermochemical Energy Storage (TCES) |
|
Gravimetric energy density |
~0.02–0.03 kWh/kg |
~0.05–0.1 kWh/kg |
~0.5–1 kWh/kg |
|
Volumetric energy density |
~50 kWh/m3 |
~100 kWh/m3 |
~500 kWh/m3 |
|
Storage temperature |
Charging step temperature |
Charging step temperature |
Room temperature |
|
Technology development |
Industrial scale |
Pilot scale |
Laboratory and pilot scale |
|
Energy storage period |
Limited (Thermal loss) |
Limited (Thermal loss) |
Theoretically unlimited |
|
Theoretical energy transport |
Very short distance |
Very short distance |
Very long distance |
|
Technology complexity |
Simple |
Medium |
Complex |
|
Drawbacks |
Important thermal losses over time Large quantity of storage material required |
Important thermal losses over time Corrosive materials Low heat conductivity |
Expensive investment cost Complex technique |
2. TCES Systems Based on Hydroxides
Ca(OH)2/CaO has been demonstrated to date to be the most interesting studied thermochemical energy storage system based on metal hydroxides and has prompted tests in lab-scale reactors and thermogravimetry analysis (TGA) (Equation (1), Figure 2) [3][7][8][9][10][11][12]. However, the enhancement of material stability is required to reduce the sintering effect. Ca(OH)2 particles were shown to agglomerate faster than CaO particles in the presence of H2O, and the presence of H2O would accelerate the agglomeration of CaO particles [13].
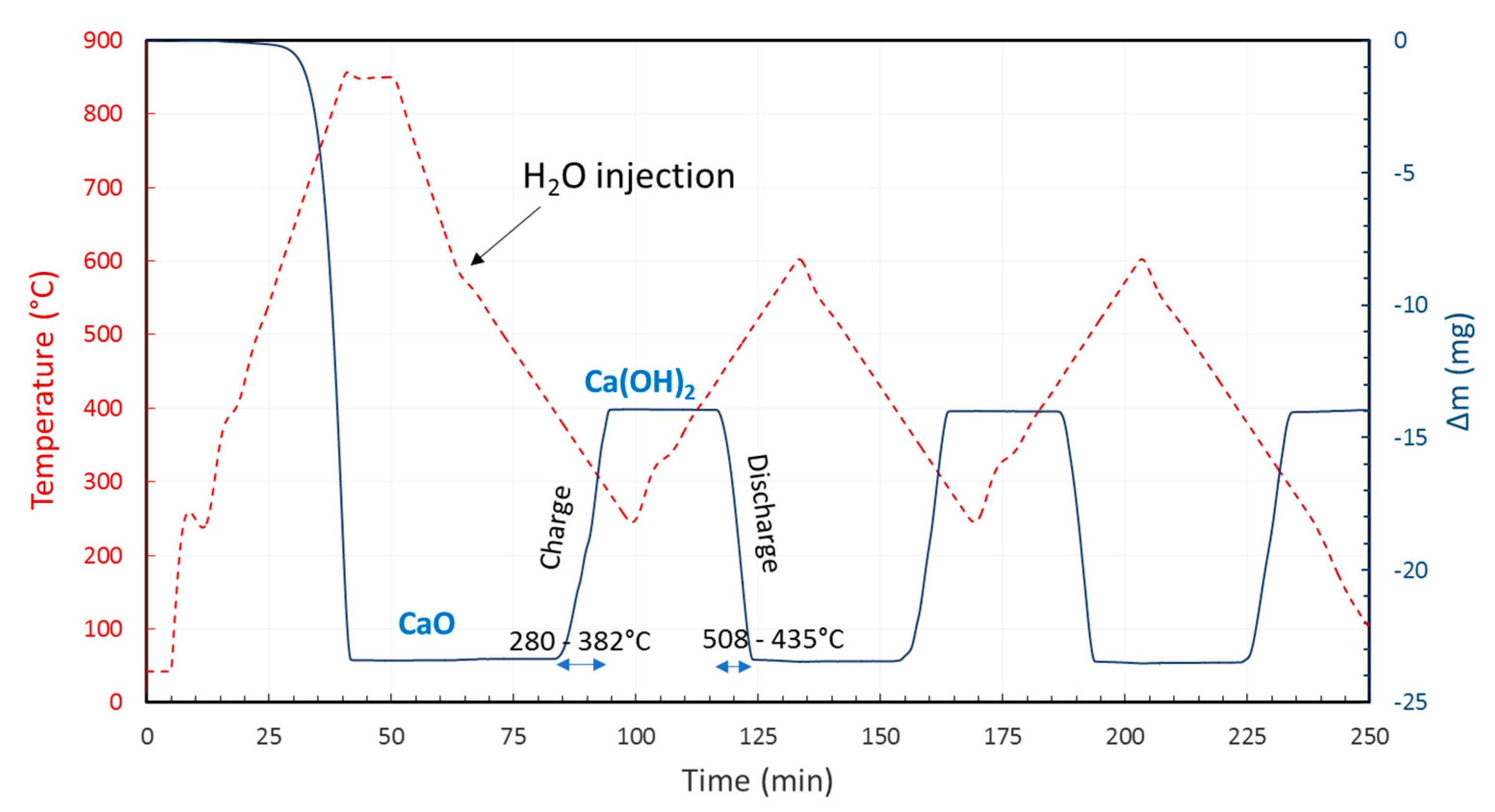
Figure 2. TGA of CaO/Ca(OH)2 showing excellent reversibility during charge/discharge cycles under 21 mol% H2O(g). CaO was first obtained from calcination of commercial CaCO3 to CaO at 850 °C under pure Ar.
The rehydration step is slower than the dehydration step [14], and the hydration of CaO agglomerated lump proved to be more difficult than that of fresh particles, which hindered the cycling stability of the material. Due to particle agglomeration, CaO/Ca(OH)2 powder bed and pellets used in packed bed reactors suffer from a loss in reactivity and a change in bulk volume. Powdered Ca(OH)2/CaO was recently evaluated under reactor conditions [15]. During the hydration/dehydration cycles, the temperature was uneven between the middle of the packed bed and the outside. During the first part of the heating, the outside of the packed bed remained at a higher temperature than the middle for at least one hour (70 min). Afterwards, the opposite was observed, with the outer part of the packed bed being at a lower temperature than the center. Under experimental conditions, the highest temperature reached was 475 °C. In fact, the study underlines the unevenness of the heat release rate and the poor thermal conductivity as main issues. To address the improvement of heat transfer through CaO/Ca(OH)2 in a packed bed reactor, a composite material using silicon carbide/silicon (SiC/Si) foam to support CaO/Ca(OH)2 in its pores (400 µm) was investigated [16]. Over ten cycles, the composite material retained a high reactivity and good stability of the bulk volume during dehydration/hydration reactions. A study was recently accomplished on CaO/Ca(OH)2 supported on ceramic honeycomb composed of silicon carbide and silicon (SiC-Si) [17]. The pellets of composite material were tested in a lab-scale packed bed reactor and were shown to enhance the heat transfer through the reaction bed. The inert honeycomb support did not form any side product during the tests; however, cracks and deformations appeared over the course of ten cycles. This approach is promising for the dispersion and shaping of packed beds using hydroxides for TCES. A CaO/Ca(OH)2/Na2CaSiO4 composite was synthesized using sodium silicate to bind CaO/Ca(OH)2 fine particles for fluidized or fixed beds [18]. This work noted the effect of the anisotropic expansion of Ca(OH)2 being the cause of the reduction in the crushing strength of the pellets.
The adaptation/integration of powdered material systems to CSP irradiated reactors such as fluidized bed is necessary for an effective exploitation of the TCES system in a continuous flow over time. To this end, calcium hydroxide was modified with nanostructured flow agents such as nanostructured silicon and/or aluminum oxide [19]. The study focused on the modification of calcium hydroxide powder using nanostructured agents, in order to enhance the flowability of the material in dynamic energy storage systems. However, the mixtures all presented lower flowability than pure Ca(OH)2/CaO powder, as the agglomeration of the pure particles led to bigger particles with better flowability. In addition, the samples from the mixture generated side products such as calcium silicate and aluminate phases which contributed to the reduction in the total heat release measured for the material. With this conclusion, it is recommended to rather improve the stability of moderately bigger particles of pure material rather than mix them with additives as a means to enhance the flowability of the material. As the low thermal conductivity and cohesiveness of powder bulk material are ill-suited for moving bed reactors, studies usually aim for granular materials for such application. To answer this issue, a recent study investigated the effect of the encapsulation of CaO granules in ceramic and of Ca(OH)2 granules coated with Al2O3 nanostructured particles [20]. Both encapsulated materials could retain their shape after six hydration/dehydration cycles, but the ceramic shell of CaO was sometimes cracked or lost. On the one hand, the reaction performances of Ca(OH)2 encapsulated in Al2O3 proved to be similar to that of unmodified Ca(OH)2 granules, but the expansion of the material during the hydration step tended to clog the reactor tubes. On the other hand, CaO granules encapsulated in ceramic flowed freely through the reactor, but their reaction performances were reduced and they could not reach full conversion. As a maneuver to answer industrial requirements—e.g., for a moving bed reactor, with appropriate material size and stability, a composite based on calcium oxide was synthesized for TCES application, using the CaO/Ca(OH)2 system [21] mixed with carboxymethyl cellulose sodium (CMC) and vermiculite. When compared to the performances of pure Ca(OH)2 tablets, the composite tablets better retained their structural integrity over several hydration/dehydration cycles. Within the material, vermiculite provided enough space for the CaO/Ca(OH)2 reaction, with an average pore diameter between 11 and 16 nm depending on the synthesis conditions for the composite material. In addition, the backbone structure of the composite possessed abundant micropores and mesopores for the gas transport during the cycles. Furthermore, the decomposition temperature of Ca(OH)2 within the composite material was reduced, which is attributed to the generation of activated carbon during the carbonization of CMC. Finally, the gravimetric storage density of the granular composite material was reduced (71% of the value obtained for pure Ca(OH)2), while the volumetric storage density was higher than that of Ca(OH)2 powder.
The enhancement of the reaction properties of the Ca(OH)2/CaO system was studied via KNO3 addition to Ca(OH)2, which reduced the dehydration reaction duration and decreased the dehydration temperature of the system due to a nitrate–hydroxide interaction as KNO3 melts during the dehydration step [22]. The influence of the amount of added KNO3 on the reaction temperature was then investigated and showed that the minimum dehydration onset temperature (459 °C, instead of 494 °C for pure Ca(OH)2) was reached with 5 wt% of KNO3 added, with the material losing only 7% of its energy storage capacity, down to 1280 kJ/kg. Additional information on the system is available, as a kinetic study of the effect of KNO3 addition to Ca(OH)2/CaO was conducted [23]. The optimum amount of KNO3 was determined to be around 10 wt% to reduce the charging temperature to 428.49 °C and accelerate the reaction with little loss in energy storage density. The cycling stability of the mixture was tested under air and under nitrogen atmosphere. The KNO3-doped calcium hydroxide fared poorly under air, due to the presence of CO2 and carbonation of the sample, but results revealed a good cycling stability under nitrogen atmosphere. SEM observations showed that within the Ca(OH)2/10 wt% KNO3 mixture, the once dehydrated material changes to a flower-like structure when rehydrated. When dehydrated again, the flower-like structure shrinks back into a blocky structure. This modification of the material morphology is attributed to the addition of KNO3 and contributes to enhancing the mass transfer during the storage cycles. Another example of reaction enhancement via doping is the improvement of reduction kinetics of Ca(OH)2 via Li doping [24]. The enhancement of the Ca(OH)2/CaO system was also approached via a modification of the structure of the material, for example with the synthesis of hexagonal boron nitride (HBN)-doped calcium hydroxide composite [25]. The material was tested in TGA/DSC (thermogravimetry analysis/differential scanning calorimetry) and showed better cycling stability than pure calcium hydroxide, showing 67% rehydration after ten dehydration/hydration cycles, with an optimum amount of HBN added at 15 wt%, and the dehydration kinetics were also enhanced. In addition, the composite material exhibited higher thermal conductivity and reaction enthalpy as compared to pure calcium hydroxide. Another studied approach to modify the properties of the system is the modification of the structure of the pure material. Ca(OH)2 nanomaterials with spindle and hexagonal structure were synthesized by a deposition-precipitation method and compared to commercial nanoparticles [26]. The spindle-shaped Ca(OH)2 demonstrated the highest specific surface area in BET and the energy storage density among the tested nanomaterials. In addition, the dehydration/hydration kinetics were improved with the spindle-shaped material and it presented the best cycling stability over ten cycles, as it retained a conversion rate above 70%.
Mg(OH)2/MgO is another potential system for TCES which is currently getting attention. Mg(OH)2 was considered at reactor scale, and an economical study was conducted [27]. However, the material suffers from slow and incomplete rehydration, as stated by Müller et al. (2019) [28]. The authors recently studied the rehydration mechanism of MgO and of natural magnesite in order to assess the effect of impurities on the reaction. The enhancement of the TCES system consisting of MgO/Mg(OH)2 was studied via the addition of LiNO3 with 1, 3, 6 and 10 wt% added [29]. The dehydration temperature of the LiNO3-Mg(OH)2 composites was lower, from 289 down to 269 °C for 1 wt% and 10 wt% doping, respectively, than that of pure Mg(OH)2 which was measured at 325 °C. The dehydration temperature of the LiNO3-Mg(OH)2 composite may then be tuned via the addition of an adequate amount of LiNO3, and the composite materials could sustain more than ten dehydration/rehydration cycles without losing thermal efficiency. In addition, the calculated dehydration rate constant was higher with LiNO3 doping, but the composite material presented lower released heat from the reaction. The mixture LiNO3/Mg(OH)2 was also studied explicitly for TCES at a lower temperature (<300 °C) since the addition of LiNO3 to Mg(OH)2 decreases the dehydration temperature of Mg-based system (76 °C difference) [30][31].
References
- Yan, Y.; Wang, K.; Clough, P.T.; Anthony, E.J. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: A review. Fuel Processing Technology 2020, 199, 106280, doi:10.1016/j.fuproc.2019.106280.
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Conversion and Management 2018, 177, 792–815, doi:10.1016/j.enconman.2018.10.011.
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484, doi:10.1016/j.energy.2017.11.084.
- Pan, Z.H.; Zhao, C.Y. Gas–solid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173, doi:10.1016/j.energy.2017.04.102.
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.; Cabeza, L. Review of Reactors with Potential Use in Thermochemical Energy Storage in Concentrated Solar Power Plants. Energies 2018, 11, 2358, doi:10.3390/en11092358.
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renewable and Sustainable Energy Reviews 2014, 32, 591–610, doi:10.1016/j.rser.2013.12.014.
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Applied Energy 2019, 254, 113733, doi:10.1016/j.apenergy.2019.113733.
- André, L.; Abanades, S. Investigation of metal oxides, mixed oxides, perovskites and alkaline earth carbonates/hydroxides as suitable candidate materials for high-temperature thermochemical energy storage using reversible solid-gas reactions. Materials Today Energy 2018, 10, 48–61, doi:10.1016/j.mtener.2018.08.007.
- Schmidt, M.; Gutierrez, A.; Linder, M. Thermochemical energy storage with CaO/Ca(OH)2 – Experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor. Applied Energy 2017, 188, 672–681, doi:10.1016/j.apenergy.2016.11.023.
- Rougé, S.; A. Criado, Y.; Soriano, O.; Abanades, J.C. Continuous CaO/Ca(OH)2 Fluidized Bed Reactor for Energy Storage: First Experimental Results and Reactor Model Validation. Ind. Eng. Chem. Res. 2017, 56, 844–852, doi:10.1021/acs.iecr.6b04105.
- Yuan, Y.; Li, Y.; Zhao, J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability 2018, 10, 2660, doi:10.3390/su10082660.
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Performance of thermochemical energy storage of a packed bed of calcium hydroxide pellets. Energy Storage 2019, 1, e40, doi:10.1002/est2.40.
- Xu, M.; Huai, X.; Cai, J. Agglomeration Behavior of Calcium Hydroxide/Calcium Oxide as Thermochemical Heat Storage Material: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 3025–3033, doi:10.1021/acs.jpcc.6b08615.
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chemical Engineering Journal 2017, 313, 1194–1205, doi:10.1016/j.cej.2016.11.010.
- Dai, L.; Long, X.-F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Applied Thermal Engineering 2018, 133, 261–268, doi:10.1016/j.applthermaleng.2018.01.059.
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Composite material for high-temperature thermochemical energy storage using calcium hydroxide and ceramic foam. Energy Storage 2019, 1, e53, doi:10.1002/est2.53.
- Funayama, S.; Takasu, H.; Kim, S.T.; Kato, Y. Thermochemical storage performance of a packed bed of calcium hydroxide composite with a silicon-based ceramic honeycomb support. Energy 2020, 201, 117673, doi:10.1016/j.energy.2020.117673.
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Solar Energy 2016, 135, 800–809, doi:10.1016/j.solener.2016.06.056.
- Gollsch, M.; Afflerbach, S.; Angadi, B.V.; Linder, M. Investigation of calcium hydroxide powder for thermochemical storage modified with nanostructured flow agents. Solar Energy 2020, 201, 810–818, doi:10.1016/j.solener.2020.03.033.
- Cosquillo Mejia, A.; Afflerbach, S.; Linder, M.; Schmidt, M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Applied Thermal Engineering 2020, 169, 114961, doi:10.1016/j.applthermaleng.2020.114961.
- Xia, B.Q.; Zhao, C.Y.; Yan, J.; Khosa, A.A. Development of granular thermochemical heat storage composite based on calcium oxide. Renewable Energy 2020, 147, 969–978, doi:10.1016/j.renene.2019.09.065.
- Shkatulov, A.; Aristov, Y. Calcium hydroxide doped by KNO3 as a promising candidate for thermochemical storage of solar heat. RSC Adv. 2017, 7, 42929–42939, doi:10.1039/C7RA06639B.
- Wang, T.; Zhao, C.Y.; Yan, J. Investigation on the Ca(OH)2/CaO thermochemical energy storage system with potassium nitrate addition. Solar Energy Materials and Solar Cells 2020, 215, 110646, doi:10.1016/j.solmat.2020.110646.
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Applied Energy 2016, 175, 277–284, doi:10.1016/j.apenergy.2016.05.038.
- Huang, C.; Xu, M.; Huai, X. Experimental investigation on thermodynamic and kinetic of calcium hydroxide dehydration with hexagonal boron nitride doping for thermochemical energy storage. Chemical Engineering Science 2019, 206, 518–526, doi:10.1016/j.ces.2019.06.002.
- Huang, C.; Xu, M.; Huai, X. Synthesis and performances evaluation of the spindle-shaped calcium hydroxide nanomaterials for thermochemical energy storage. J Nanopart Res 2019, 21, 262, doi:10.1007/s11051-019-4694-z.
- Flegkas, S.; Birkelbach, F.; Winter, F.; Groenewold, H.; Werner, A. Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies 2019, 12, 4788, doi:10.3390/en12244788.
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration – From fundamentals to phase impurities. Applied Energy 2019, 253, 113562, doi:10.1016/j.apenergy.2019.113562.
- Li, S.; Liu, J.; Tan, T.; Nie, J.; Zhang, H. Optimization of LiNO3–Mg(OH)2 composites as thermo-chemical energy storage materials. Journal of Environmental Management 2020, 262, 110258, doi:10.1016/j.jenvman.2020.110258.
- Shkatulov, A.I.; Aristov, Y. Thermochemical Energy Storage using LiNO3-Doped Mg(OH)2: A Dehydration Study. Energy Technology 2018, 6, 1844–1851, doi:10.1002/ente.201800050.
- Shkatulov, A.; Takasu, H.; Kato, Y.; Aristov, Y. Thermochemical energy storage by LiNO3-doped Mg(OH)2: Rehydration study. Journal of Energy Storage 2019, 22, 302–310, doi:10.1016/j.est.2019.01.014.
 Encyclopedia
Encyclopedia


