Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Pediatrics
The Crohn’s Disease Exclusion Diet (CDED) is a new generation of nutritional therapy—in its initial stages, aimed at inducing remission, it involves a combination of partial enteral nutrition (PEN) with selected natural diet products. The primary mechanism of action is to exclude or limit exposure to dietary factors with potentially deleterious effects on the pathogenesis and course of CD.
- Crohn’s disease
- guidelines
- induction of remission
- nutritional treatment
- elimination diet
1. Introduction
The etiology of CD is unknown. Currently, interactions between environmental factors and the intestinal microflora and immune system (intestinal barrier) in individuals with a genetic predisposition to develop the disease are considered the most likely pathomechanism (Figure 1) [1]. The critical importance of environmental factors is confirmed by epidemiological data, including incidence rates around the world. Interesting information was provided by observations among people who moved from regions with low incidence to countries with high incidence—it was shown that the offspring of immigrants had the same risk of CD as children coming from families living in regions with high incidence for many generations [2]. One of the best-studied environmental factors in the context of CD pathogenesis is diet. Recent data suggest that as a result of specific dietary factors, the delicate immune balance between the microbiota and the intestinal mucosa is disrupted, leading to chronic inflammation. This hypothesis is supported by research findings that show a correlation between the so-called Western (industrialised) dietary pattern and increased risk of CD [3][4][5].
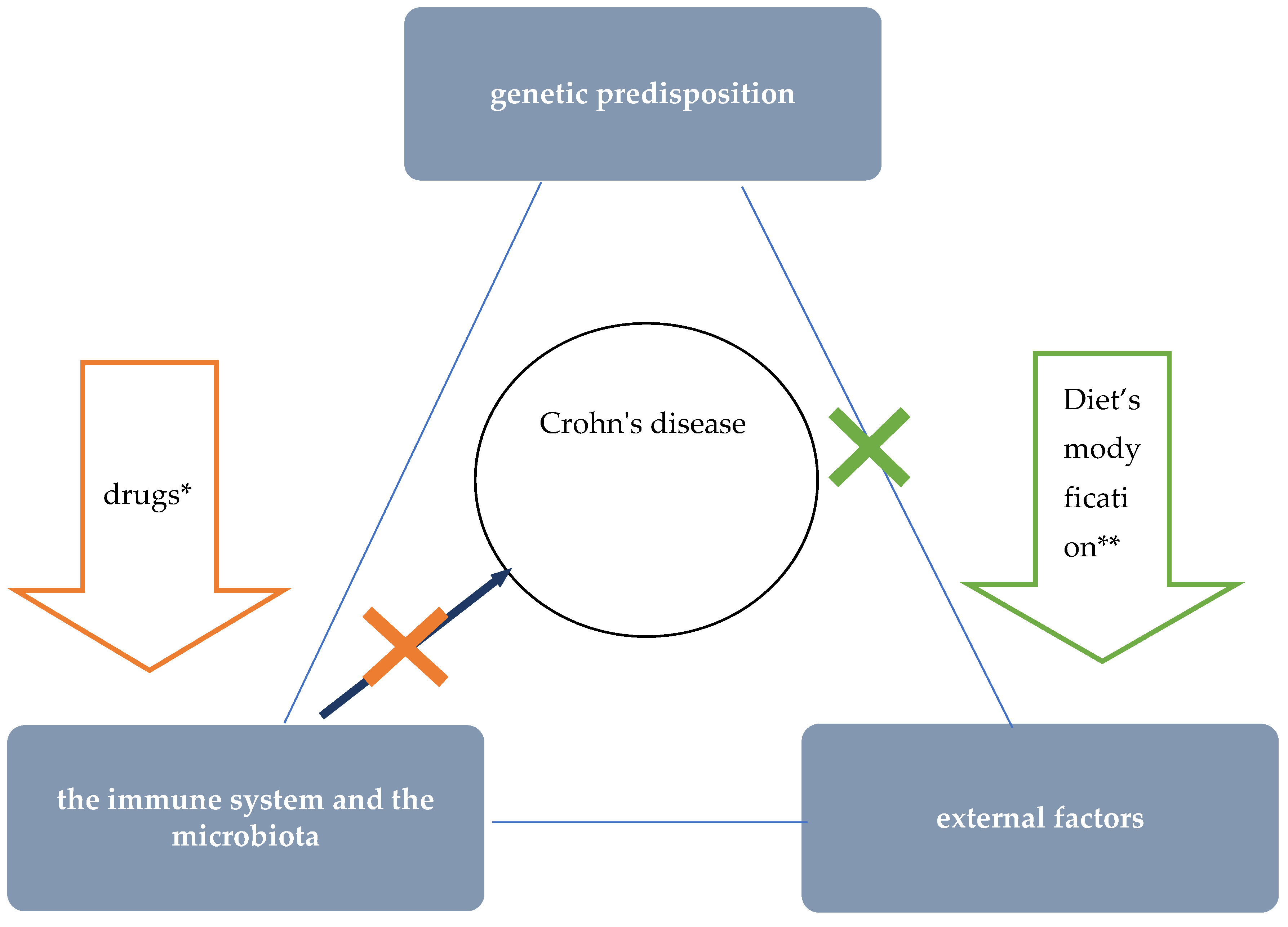 Figure 1. The role of nutritional therapy in the treatment of Crohn’s disease. * steroids, immunosuppressants, and biologics’ ** such as EEN.
Figure 1. The role of nutritional therapy in the treatment of Crohn’s disease. * steroids, immunosuppressants, and biologics’ ** such as EEN.Epidemiological studies proved the influence of the Western diet on an increased incidence of CD and, conversely, a lower incidence among those following a Mediterranean diet [3][5]. One of the features typical of the Western dietary pattern is the high consumption of processed foods. Meanwhile, a number of adverse consequences resulting from exposure to a variety of food additives were demonstrated in studies in cellular and animal models [3]. For example, the destructive effects of emulsifiers (i.e., carboxymethylcellulose and polysorbate-80) on the mucus layer that protects intestinal epithelial cells was proved [6][7][8]. Due to their gelling and thickening properties, these additives are commonly used in meat and dairy products, among other things. Another problem specific to the Western diet is low dietary fibre intake. Particularly relevant to the pathogenesis of CD may be a low supply of food that provides substrates for the production of short-chain fatty acids (SCFAs), especially water-soluble dietary fibre and resistant starch [9]. Short-chain fatty acids, which are a metabolic product of the bacteria residing in the intestines, play an important role in maintaining normal intestinal barrier and immune system function in a number of ways, including:
-
by influencing the activity of immune cells and their migration to the site of inflammation, they exhibit anti-inflammatory effects;
-
by nourishing colonocytes, providing them with a primary source of energy;
-
by reducing the pH in the intestine, positively influencing the composition of the intestinal microbiota (stimulating the growth of beneficial strains of bacteria and inhibiting the growth of pathogenic bacteria).
On the other hand, in the absence of sources for SCFA production, the mucus layer that protects intestinal epithelial cells can be used by bacteria as a medium, allowing their translocation through the intestinal mucosa [9][10].
Other features of the Western diet with potential negative effects on CD development include high consumption of red/processed meat, animal fat, and wheat (Figure 2) [3]. For example, studies in animal models proved, among other things, the effect of a diet high in fat and sugars on adverse changes in the composition of the intestinal microbiota and on impaired expression of short-chain fatty acid receptors (GPR43). GPR43 are involved in mechanisms regulating SCFA-mediated immune responses [11].
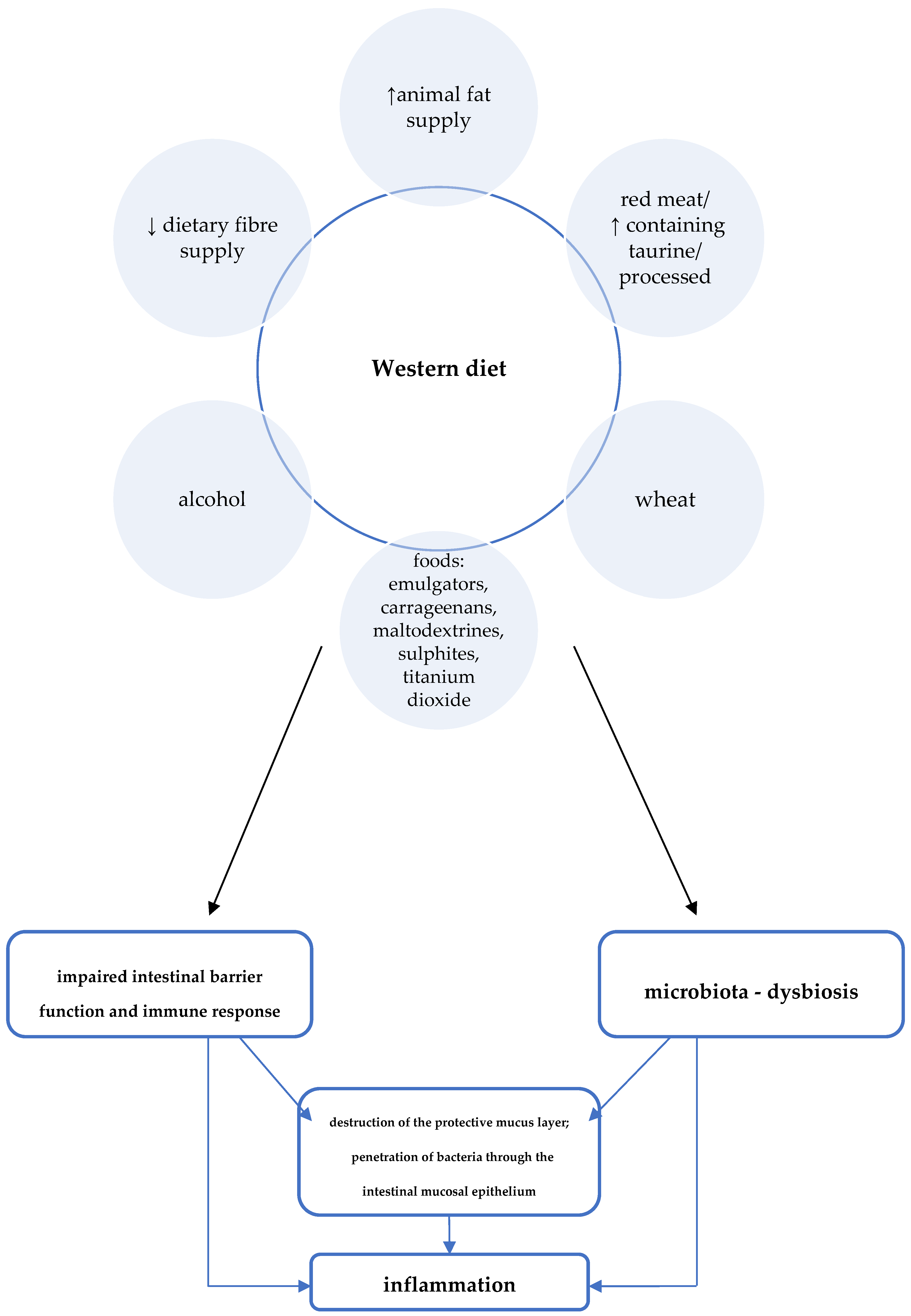 Figure 2. Western diet and the pathogenesis of Crohn’s disease [3].
Figure 2. Western diet and the pathogenesis of Crohn’s disease [3].A summary of Western dietary factors considered as particularly important in the pathomechanism of inflammation in CD is shown in Figure 2.
2. CDED—Protocol and Mechanism of Action
The CTable 1 presents the major food groups eliminated in the Crohn’s Disease Exclusion Diet.
Table 1. Major food groups eliminated in the Crohn’s Disease Exclusion Diet.
| Natural Products—Exclusion or Controlled Exposure | Food Additives—Exclusion |
|---|---|
|
|
The direct effect of the elimination diet on restoring eubiosis, normal intestinal barrier function, and immune response, causes the quenching of inflammation and healing of the intestinal mucosa (Figure 3).
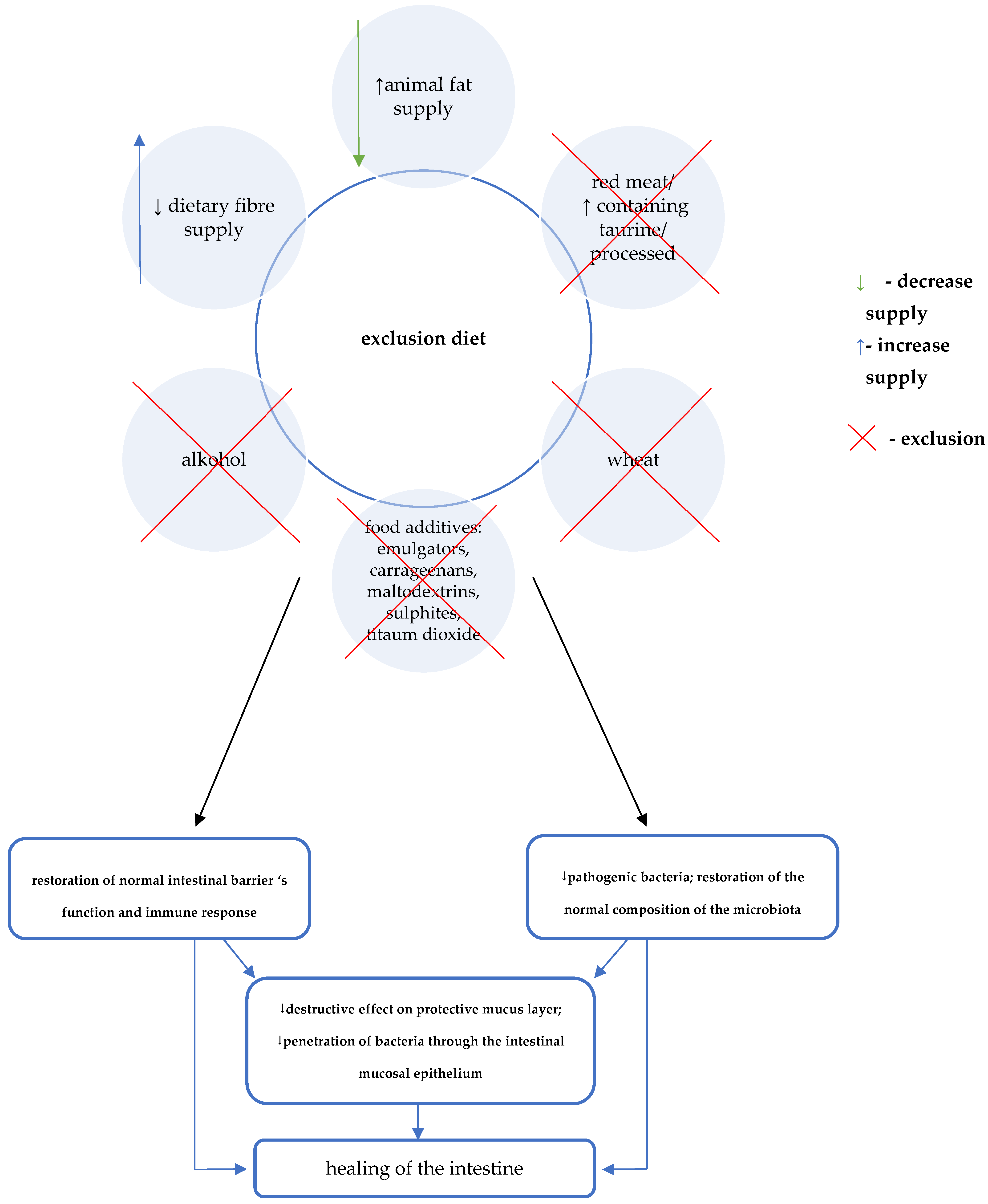 Figure 3. Crohn’s Disease Exclusion Diet (CDED) diet and the treatment of Crohn’s disease [12][13][14].
Figure 3. Crohn’s Disease Exclusion Diet (CDED) diet and the treatment of Crohn’s disease [12][13][14].The auxiliary mechanism of CDED involves consideration of the supply of specific components that may provide additional benefits [12][13][14]. A key role in this regard is attributed to the effect of increasing the production of short-chain fatty acids. Therefore, foods that are mandatory in CDED include selected foods rich in water-soluble fibre (e.g., pectin) as well as resistant starch (Table 2) [3][12][13][14].
Table 2. Foods mandatory/allowed during the induction phase of CDED (stages 1 and 2).
| Mandatory Foods (Stages 1 and 2) | ||
|---|---|---|
| A balanced diet—source of complete protein with low taurine content | Additional benefits—sources of water-soluble dietary fibre (pectins) and resistant starch | |
|
|
|
| Group of foods | allowed in stage 1 (weeks 1—6) |
Additionally allowed in stage 1 (weeks 1—6) *** |
| Cereals | white rice, rice flour and rice pasta (in unlimited amounts) | - quinoa (in unlimited amounts) - sweet potato (½ can replace 1 potato, once a day) - oatmeal—½ cup 1—2 times a week (can be used to make oatmeal or oatmeal cookies) - one slice of whole-grain bread/day (without yeast) |
| Meat/fish/eggs | instead of chicken breast, a serving of lean white fish once a week. | - fresh lean beef (lean meat i.e., sirloin) can be eaten once a week instead of chicken breast - a serving of tuna in canola oil or olive oil once a week |
| Vegetables | - 2 tomatoes or 6 cherry tomatoes - 2 peeled cucumbers - 1 young carrot - fresh spinach (a cup once a day) - lettuce leaves (3 per day) - avocado (1 a day; max. ½/meal) |
gradual introduction of new vegetables: - initially those containing lower amounts of dietary fibre (e.g., 1 small zucchini, 2 broccoli or cauliflower florets) - starting from week 10 other vegetables can br introduced (except for cabbage, leak, asparagus, artichoke and celery)—e.g., ½ of sweet red bell pepper, beet |
| Fruits | - strawberries (several a day) - a slice of melon |
gradual introduction of new fruit: in weeks 7–9 1 pear, peach or kiwi/a day can be eaten—instead of a serving of strawberries, 10 bilberries (or a cup) can be eaten - form week 10 other fruits can be introduced (which contain more fibre), but in limited quantities—i.e., ½ cup of mango, pineapple cubes or orange slices (except for passion fruit, pomegranate, cactus, kaki) |
| Fats | - olive oil - canola oil |
|
| Sugar and sweets |
- honey (3 teaspoons a day) or - sugar (4 teaspoons a day) |
|
| other | - legumes (lentils, chickpeas, beans, peas)—½ cup dry seeds a day) - almonds or walnuts (unsalted, unroasted, unprocessed)—8 pieces a day - tahini (without emulsifying agents and sulphites)—2 spoons a day |
|
| fluids | - water (slices of lemon, lime, orange or mint leaves can be added to taste) - herbal teas (preferable from fresh leaves) - 1 glass of freshly squeezed orange juice |
|
| Spices | -salt, pepper, paprika, cinnamon, cumin, turmeric - fresh herbs i.e.: mint, oregano, cilantro, rosemary, sage, basil, thyme, dill, parsley - other spices: onion, garlic, ginger, fresh lemon juice |
|
* preferably boiled and cooled. ** preferably medium mature. *** as well as all foods allowed during stage 1. The protocol of the CDED is divided into three phases, including 2 (lasting 6 weeks each) stages of the induction stage and phase 3—maintenance, which should be continued for at least 9 months, and treated as the target diet. Each subsequent phase is less restrictive and easier.
In the first phase, due to the greatest restrictions regarding the allowed foods, 50% of daily energy demand must be satisfied by enteral diet. Phase 2 is the time of reintroduction of some foods that had to be eliminated during the first 6 weeks. Due to the increasing variety of foods that are allowed, the recommended % of energy provided with the formula is reduced to 25% of the demand. Satisfying some nutritional needs with the use of a complete enteral diet is aimed at preventing deficiencies and maintaining/improving the nutritional status in the period of the greatest restrictions, i.e., in the first 2 phases of the diet. The principles of formula selection are the same as the guidelines for exclusive enteral nutrition in children. Standard polymeric, normocaloric (1 kcal/1 mL) diets are recommended. In justified cases, that is in patients with food intolerances, allergy to cow’s milk proteins or insufficient tolerance of the polymeric preparation, preparations containing hydrolysed protein should be recommended.
Foods that can be consumed in the first two phases of the CDED are divided into foods that are:
- –
-
mandatory, i.e., recommended for daily consumption. Their role is to provide adequate nutritional value of the diet as well as substrates for the production of SCFA;
- –
-
neutral, which are supposed to add variety to the daily menu, but do not necessarily have to be consumed.
- –
-
forbidden.
Table 2 presents the summary of mandatory and neutral products that can be consumed during phases 1 and 2 of the diet. The quantity of enteral feeding and particular supplementing products should be determined individually, in accordance with the state of nutrition stemming from sex, age and activity of the disease as well as energy and nutrient demand.
In phase 3 (maintenance), patients should function according to the principle of controlled exposure to dietary components with potentially negative effects on pathogenesis and the course of the disease. Therefore, for five days a week, they should compose their meals based on products allowed in phase 2 as well as selected additional products. During these days, especially in the case of patients who did not normalise the parameters of their nutritional status despite good treatment effects, it is recommended that supplementation with formula be continued. In addition, on selected consecutive days it is possible to eat two meals composed of products that are not recommended for daily consumption. Patients should still avoid particularly harmful foods, mainly highly processed products, i.e., processed meats, frozen, ready-to-eat foods and sweetened beverages. Table 3 presents a summary of foods/meals that can be consumed additionally during the maintenance phase of CDED.
Table 3. Foods allowed in the maintenance phase of CDED.
| Foods Allowed 5 Days a Week * | Examples of Products Allowed 2 Days a Week ** | |
|---|---|---|
| Examples of Foods That Can Be Consumed for Breakfast: | Examples of Foods That Can Be Consumed for Lunch (Dinner): | |
| - other parts of chicken (except for skin or giblets) - fresh seafood, salmon (once a week)—1 serving of unprocessed, full-fat natural yoghurt (without additives) a day - 2 slices of whole-grain bread (without yeast), or 1 serving of pasta a day - all vegetables except for leak, celery stalks and large amounts of kale - all fruits (including dried fruits, without sulphites) except for passion fruit, pomegranate, cactus, kaki - 1 cup of black coffee (not instant) or tea |
- any kind of bread - milk, cheese - crêpes - jams - 1 bowl of cereal with milk |
- steaks, burgers, pork, seafood, fish, i.e., salmon and tuna, - any type of pasta - dairy products, including cheese - 1 serving of home-made dessert (e.g., cake) or one scoop of ice-cream - cocoa/dark chocolate |
| - or 1 meal eaten out instead of unrestricted meals prepared at home | ||
* as well as all foods allowed in phase 2. ** can be eaten for 2 selected meals during the day.
3. Summary
As of today, EEN is the sole method of nutritional treatment with proven efficacy in inducing remission in children with active Crohn’s disease that is recommended in the official guidelines. This form of dietary treatment, however, has significant limitations that have a negative impact on the possibility of its wide use and long-term maintenance of its positive effects. It seems that the groundbreaking reports concerning CDED, a modern method of dietary treatment, which indicates that it is at least comparable with EEN in terms of its effectiveness in inducing remission and that is much better tolerated by patients, CDED will be included in the latest guidelines of scientific societies.
This entry is adapted from the peer-reviewed paper 10.3390/jcm10143027
References
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 2019, 156, 1354–1367.
- Charlebois, A.; Rosenfeld, G.; Bressler, B. The impact of dietary interventions on the symptoms of inflammatory bowel disease: A systematic review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1370–1378.
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738.
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012, 487, 104–108.
- Khalili, H.; Hakansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, W. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’sdisease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644.
- Roberts, C.L.; Rushworth, S.L.; Richman, E.; Rhodes, J.M. Hypothesis: Increased consumption of emulsifiers as an explanation for the rising incidence of Crohn’s disease. J. Crohns Colitis 2013, 7, 338–341.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96.
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427.
- Lewis, J.D.; Abreu, M.T. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 398–414.
- Czajkowska, A.; Szponar, B. Krótkołańcuchowe kwasy tłuszczowe (SCFA) jako produkty metabolizmu bakterii jelitowych oraz ich znaczenie dla organizmu gospodarza. Postepy. Hig. Med. Dosw. 2018, 72, 131–142.
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive, E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032.
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s Disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360.
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Avraham, S.B.; Mona Boaz, M.; Levine, A. Dietary therapy with the Crohn’s Disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J. Crohns Colitis 2017, 11, 1205–1212.

