Factors secreted from adipose tissue may induce and/or maintain a local and systemic low-grade activation of the innate immune system. Attraction of macrophages into adipose tissue and altered crosstalk between macrophages, adipocytes, and other cells of adipose tissue are symptoms of metabolic inflammation. Among several secreted factors attracting immune cells to adipose tissue, chemotactic C-C motif chemokine ligand 2 (CCL2) (also described as monocyte chemoattractant protein-1 (MCP-1)) has been shown to play a crucial role in adipose tissue macrophage infiltration.
- CCL2
- Adipose Tissue Inflammation
- Chemokines
- Adipokine
1. Introduction
Accumulation of adipose tissue (AT) is the major symptom of obesity. Until about 25 years ago, AT was regarded as an energy storage organ that additionally acts as isolation for the inner organs [1]. Due to the discovery of its endocrine function in the late 1980s, our understanding of AT changed fundamentally [2]. Since then, hundreds of bioactive components secreted by AT have been found [3][4]. Those AT-derived secretory factors including leptin, adiponectin, adipsin, plasminogen activator inhibitor-1 (PAI1), complement components, or cytokines such as tumor necrosis factors (e.g., TNF-α) or chemokines (e.g., CCL2) have been described with the term “adipokines” [5].
In 1999, Funahashi et al. defined “biologically active molecules secreted from adipose tissue” as “adipocytokines” [6]. However, this term is potentially misleading because it suggests that all AT-secreted substances are cytokines. While this is true for some AT-secreted molecules (e.g., IL-6 or TNF-α), the majority is of non-cytokine origin. Although Trayhurn and Wood recommended to use the term “’adipokine’ […] to describe a protein that is secreted from […] adipocytes”, commonly all AT-produced and -secreted substances are named “adipokines”, independent of whether they are secreted primarily from adipocytes or other AT cell types [7].
Adipokines are a heterogenous group of peptides including hormones, growth factors, and cytokines which differ in their physiological functions. Adipokines play an important role in the regulation of energy homeostasis, appetite, satiety, lipid metabolism and glucose homeostasis, blood pressure and vascular homeostasis, angiogenesis, and immune response [8]. Whereas adipocyte-secreted adiponectin and leptin circulate in the blood as endocrine factors, it was demonstrated that some adipokines mainly have a para or autocrine functions within AT without a contribution to inter-organ tissue crosstalk [9]. Serum concentrations of several adipokines reflect body energy stores, fat mass and distribution, systemic insulin sensitivity, glucose tolerance, a pro-inflammatory state, and other phenotype characteristics [4][10][11][12][13][14][15][16]. As examples, leptin serum concentrations are proportionally secreted to body fat mass [17], where circulating adiponectin are typically lower in individuals with obesity compared to those who are lean [18]. Additionally, several immune-modulating adipokines, such as IL-6, IL-8, CXCL5, or CCL2, are elevated in the obese state [9][19]. These changes in adipokines’ secretion pattern can be explained by AT remodeling, a process in which quantitative and qualitive changes in the cellular composition of AT occur in response to excessive weight gain [20].
AT is a complex organ composed of several cell types (Figure 1). Adipocytes account for up to 80% of AT volume, but reflect only 20–40% of cell number. AT consists of adipose-derived stem cells (ADSCs), preadipocytes, endothelial cells, and leukocytes [21][22]. Very recently, single-nucleus RNA-sequencing (snRNA-seq) analysis of mouse and human adipose tissue revealed a subpopulation of adipocytes that regulates thermogenesis [23]. Depending on their type, different AT-cells produce distinct adipokine patterns. Therefore, knowing the cellular origin for adipokine production is important to dissect which cell type might be enriched and/or activated in AT. For example, adipocytes exhibit a quantitatively distinct adipokine pattern in the function of the fat-depot (subcutaneous (sc) and visceral (vis)) origin. Adiponectin and leptin are predominantly expressed in sc AT [24]. In contrast, IL-6 [25], omentin [26], visfatin [27], and RBP4 exhibit higher vis than sc production [28]. Adipsin [29], lipocalin 2 [30], and TNF-α [31] are secreted in both depots in comparable amounts. Using single-cell or single nuclei transcriptomics, it is now possible to discriminate adipocyte subpopulations within one depot, as well as more than 10 different AT cell types which differ in their metabolic and transcriptional properties including the identification of differences in cellular adipokine sources [23][32][33][34].
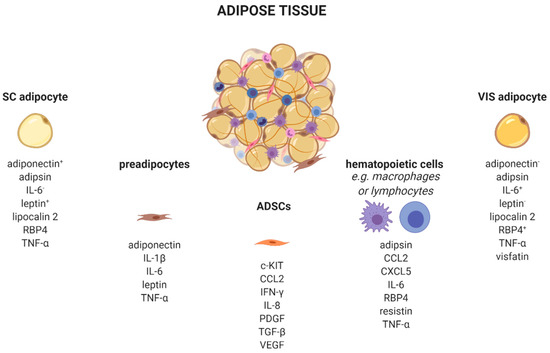
Figure 1. Adipose tissue cells secrete distinct adipokines. Adipose tissue consists of a variety of cell types, such as adipocytes, preadipocytes, adipose tissue-derived stem cells, and several immune cells which produce and secrete cell-type-specific adipokines. +/−, higher/lower secreted in sc or vis. c-KIT, KIT proto-oncogene, receptor tyrosine kinase; CCL2, C-C motif chemokine ligand 2; CXCL5, C-X-C motif chemokine ligand 5; IFN-γ, interferon-γ; IL, interleukin; PDGF, platelet derived growth factor; RBP4, retinol binding protein 4; SC, subcutaneous; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor; VIS, visceral. Created with BioRender.com.
Besides adipocytes, ADSCs produce a variety of chemokines and growth factors such as the pro-angiogenic CCL2, IL-8, vascular-endothelial growth factor (VEGF) [35], platelet-derived growth factors (PDGF) [36], or c-kit which induces endothelial cell proliferation [37]. Furthermore, ADSCs secrete immune-modulating substances like interferon-γ (IFN-γ) or transforming growth factor-β (TGFβ) [38].
Depending on the fat depot, 15–50% of all resident AT-cells are preadipocytes [39]. Using a conditioned medium from obese murine epididymal AT, Renovato–Martins et al. demonstrated that 3T3-L1 preadipocytes secrete leptin and adiponectin as well as the pro-inflammatory factors IL-6, TNFα, and IL-1β [40].
In addition to those cell types of mesenchymal origin, there are various hematopoietic cells resident in AT. Nearly all known leukocytes such as macrophages, monocytes, dendritic, or natural killer cells, B-, and T-cells, as well as neutrophils or eosinophils, are of high importance in the adipokine context. The majority of immune cells express the leptin receptor on their cellular surface. Since circulating levels elevate proportional to the amount of white adipose tissue, leptin acts as a pro-inflammatory adipokine on immune cells. Subsequently, leptin receptor signaling via JAK2-STAT leads to a broad range production of pro-inflammatory adipokines, such as interleukins (IL-6, IL-8, IL-12, and IL-18), TNFα, or CCL2 [22][41]. Indeed, CCL2 (MCP-1) is a member of the small inducible gene family that plays a role in the recruitment of monocytes to sites of injury and infection, but also to AT under conditions of inflammation or adipocyte apoptosis [42][43][44]. Recently, CCL2 has been described as an important factor linking sc AT to altered glucose metabolism and body fat distribution [45].
2. C-C Motif Chemokine Ligand 2
2.1. Structure, Sources and Signaling
Chemokines are proteins with molecular weights ranging between 8 to 12 kDa that mediate cellular movement (chemotaxis), hematopoiesis, leukocyte degranulation, and angiogenesis [46]. Four chemokine subfamilies have been categorized based on the number and location of N-terminal cysteine residues: C, CC, CXC, and CX3C [47]. Chemokine sequences are highly conserved and share similar structures consisting of a flexible N-terminus followed by a loop containing three antiparallel β-sheets on to which is folded an α-helix [48]. Experiments which also defined the crystal structure of CCL2 revealed that it forms dimers in an anti-parallel β strand arrangement between the two flexible N-termini [49].
CCL2, also known as monocyte chemoattractant protein-1 (MCP-1), was the first discovered human CC-family chemokine [50][51]. The gene is located on chromosome 17 (q11.2) [52] and is produced by endothelial cells, fibroblasts, epithelial, smooth muscle, mesangial, astrocytic, monocytic, and microglial cells [53][54][55][56], whereas monocytes and macrophages are major sources of CCL2 [57][58]. Additionally, (pre-)adipocytes express CCL2 [59].
CCL2 expression is induced by inflammatory stimuli (IL-1, IL-4, IL-6, tumor necrosis factor α (TNFα)), transforming growth factor β (TGFβ), lipopolysaccharide (LPS), interferon γ (IFNγ), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), macrophage colony-stimulating factor (M-CSF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [60]. Human serum CCL2 has been associated with a chronic pro-inflammatory state and was suggested as a biomarker for malignant disease such as prostate and breast cancer [61][62]. High CCL2 expression in tissues indicates chemo-attraction of monocytes in the context of local defense mechanism activation and repair of tissue damage [63].
Chemokines are secreted in response to pro-inflammatory signals, such as cytokines, to selectively recruit immune cells including monocytes, neutrophils, or lymphocytes to sites of inflammation or injuries. For CCL2, there are two activation pathways described. During the canonical pathway, inflammatory substances such as tumor-necrosis factor α (TNFα) binds to its receptor, resulting in the activation of the inhibitor of nuclear factor-κB kinase (IKK). Activated IKK then phosphorylates the NF-κB-bound inhibitor of NF-κB (IκB), whereby IκB is degraded. Consequently, released NF-κB homodimers translocate to the nucleus where they activate the transcription of inflammation-related genes e.g., CCL2, TNFα, and IL-6 [64]. Alternatively, CCL2 can be activated by the non-canonical pathway, i.e., NF-κB-independent CCL2 expression stimulated by PDGF [50] or insulin. In human aortic endothelial cells, physiological insulin concentrations were shown to suppress the expression of both NF-κB and CCL2 by more than 60% [65]. Nakatsumi et al. demonstrated that insulin activates the phosphatidylinositol 3-kinase (PI3K)-Akt pathway which leads to the inhibition mTORC1-repressor, the ras homolog enriched in brain (RHEB). In turn, mTORC1 induces forkhead box K1 (FOXK1) dephosphorylation via protein phosphatase 2A (PP2A), leading to CCL2 expression [66] (Figure 2).
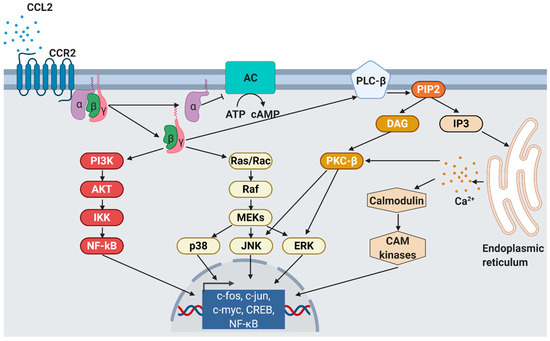
Figure 2. CCL2 signaling. As response to CCL2 binding at the N-terminus, extracellular loops and transmembrane bundle of CCR2, the intracellular G-protein αi subunit dissociates from the CCR2 and the βγ subunit. The α subunit then inhibits adenylyl cyclase (AC) function resulting in decreased cyclic adenosine monophosphate levels. In contrast, the βγ subunit signaling induces gene expression via several pathways. PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; IKK, inhibitor of NF-κB kinase; NF-κB, nuclear factor of kappa-light-chain-enhancer of activated B cells; Ras, rat sarcoma; Rac, ras-related C3 botulinum toxin substrate; Raf, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase; p38, mitogen-activated protein kinase; JNK, c-jun N-terminal kinase; ERK, extracellular signal-regulated kinase; PLC-β, phospholipase C-β; PIP2, phosphatidylinositol 4,5-bisphosphate; DAG, diacylglycerol; IP3, inositol 1,4,5-triphosphate; PKC-β, protein kinase C-β; CAM, Ca2+/calmodulin-dependent protein kinase; c-fos, proto-oncogene c-Fos; c-jun, proto-oncogene Jun; c-myc, proto-oncogene Myc; CREB, cAMP response element-binding protein. Modified from Bose S. & Cho J. [67] using BioRender.com.
The effects of CCL2 on target cells are mediated by a specific chemokine receptor. Cells that express the distinct CC chemokine receptor (CCR) are able to migrate along the chemokine gradient upon CCL2 activation [68]. CCRs are G-protein coupled receptors (GPCRs) belonging to the rhodopsin or serpentine receptor family [69] which are expressed on different types of leukocytes such as eosinophils, basophils, lymphocytes, macrophages, and dendritic cells [70]. Human CC chemokines bind to at least two different CCRs.
CCL2 usually binds to CCR2 that exists in two different splice variants, CCR2A and CCR2B, which differ in their C-terminal tails [71]. In contrast to the widespread expression of CCL2, CCR2A is mainly expressed by vascular smooth muscle cells and mononuclear cells, whereas CCR2B is the predominant receptor on monocytes and natural killer cells [72]. In addition to CCL2, CCR2 binds another five pro-inflammatory cytokines, CCL7 [73], CCL8 [74], CCL12 [75], CCL13 [67], and CCL16 [76]. However, CCL2 has the highest activation potential that finally leads to monocyte migration into target tissues [77]. As a result of CCL2/CCR2 binding, cell migration is promoted by the activation of several signaling cascades such as JAK2/STAT3 [78], MAP kinase [79], and PI3K [80] pathways (Figure 3).
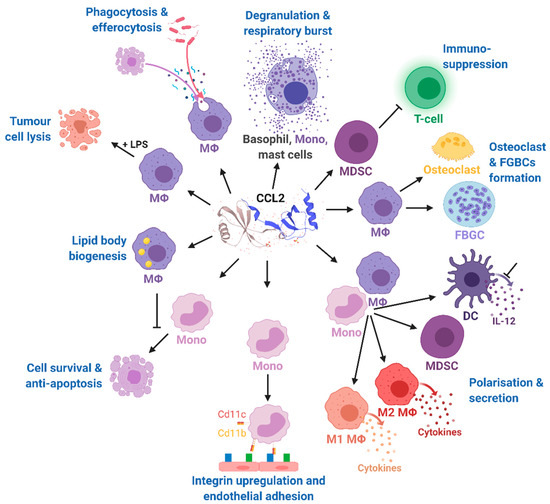
Figure 3. Effects of CCL2 on different immune cell types. Mono, monocytes; MΦ, macrophages; DC, dendritic cells; FBGCs, foreign body giant cells; MDSC, myeloid-derived suppressor cell; IL-12, interleukin 12; LPS, lipopolysaccharide; CD11b, cluster of differentiation molecule 11B; CD11c, cluster of differentiation molecule 11C. The CCL2 structure was taken from uniprot [81]. Modified from Gschwandtner M. et al. [60] using BioRender.com.
CCL2’s binding at CCR2 results in the dissociation of GDP from the Gαi subunit which associates with intracellular GTP and inhibits membrane-bound adenylyl cyclases, finally leading to decreased intracellular cAMP levels. In contrast, the released G-protein βγ heterodimer activates phospholipase C which then hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) [67]. IP3 diffuses in the cytosol and stimulates calcium release from the endoplasmic reticulum [82]. The released Ca2+ is further bound by calmodulin (CaM), an essential modulator of various processes like immune response, inflammation [83], apoptosis, or metabolism [84]. Elevated Ca2+ levels as well as DAG activate protein kinase C-β (PKC-β) that mediates gene expression via c-Jun N-terminal kinases (JNK) and extracellular signal-regulated kinases (ERK) activation [85]. Monocyte migration is regulated via Gβγ activation of PI3K/Akt, which in turn polymerizes actin for pseudopod formation [86].
The multiple and pleiotropic effects of CCL2 on multiple cells of the myeloid lineage are summarized in Figure 4 and are more extensively discussed in a recent comprehensive review highlighting the role of CCL2 on immune cell behavior and tumor immunity [60]. In our review, we focused on the role of CCL2 in the context of obesity-related diseases.
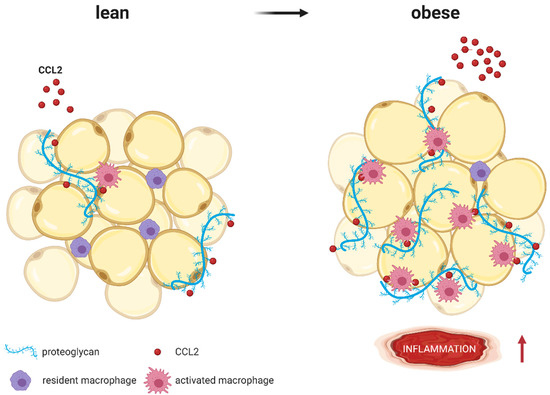
Figure 4. CCL2 (red molecules) binds to proteoglycans (blue strands) such as heparan sulfate or heparin, which are part of the extracellular matrix surrounding adipocytes. Whereas lean AT expresses low proteoglycan levels, expression increases with obesity. As coreceptors, proteoglycans immobilize CCL2 and present it to macrophages, resulting in higher AT inflammation (represented by ↑). Modified from Pessentheiner A. et al. [87] using BioRender.com.
References
- Obregon, M.-J. Adipose tissues and thyroid hormones. Front. Physiol. 2014, 5, 479.
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556.
- Lehr, S.; Hartwig, S.; Lamers, D.; Famulla, S.; Müller, S.; Hanisch, F.-G.; Cuvelier, C.; Ruige, J.; Eckardt, K.; Ouwens, D.M.; et al. Identification and validation of novel adipokines released from primary human adipocytes. Mol. Cell. Proteomics 2012, 11, M111.010504.
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524.
- Arner, P. Editorial: Visfatin—A True Or False Trail To Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 28–30.
- Funahashi, T.; Nakamura, T.; Shimomura, I.; Maeda, K.; Kuriyama, H.; Takahashi, M.; Arita, Y.; Kihara, S.; Matsuzawa, Y. 3. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern. Med. 1999, 38, 202–206.
- Trayhurn, P.; Wood, I.S. Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004, 92, 347–355.
- Khan, M.; Joseph, F. Adipose Tissue and Adipokines: The Association with and Application of Adipokines in Obesity. Scientifica 2014, 2014, 28592.
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282.
- Ebert, T.; Gebhardt, C.; Scholz, M.; Wohland, T.; Schleinitz, D.; Fasshauer, M.; Blüher, M.; Stumvoll, M.; Kovacs, P.; Tönjes, A. Relationship between 12 adipocytokines and distinct components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 2018, 103, 1015–1023.
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470.
- Flehmig, G.; Scholz, M.; Klöting, N.; Fasshauer, M.; Tönjes, A.; Stumvoll, M.; Youn, B.S.; Blüher, M. Identification of adipokine clusters related to parameters of fat mass, insulin sensitivity and inflammation. PLoS ONE 2014, 9, e99785.
- Fasshauer, M.; Blüher, M.; Stumvoll, M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2014, 2, 488–499.
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515.
- Ebert, T.; Roth, I.; Richter, J.; Tönjes, A.; Kralisch, S.; Lossner, U.; Kratzsch, J.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Different associations of adipokines in lean and healthy adults. Horm. Metab. Res. 2014, 46, 41–47.
- Tönjes, A.; Fasshauer, M.; Kratzsch, J.; Stumvoll, M.; Blüher, M. Adipokine pattern in subjects with impaired fasting glucose and impaired glucose tolerance in comparison to normal glucose tolerance and diabetes. PLoS ONE 2010, 5, e13911.
- Ahima, R.S. Revisiting leptin’s role in obesity and weight loss. J. Clin. Investig. 2008, 118, 2380–2383.
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res. Int. 2014, 2014, 658913.
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97.
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 1.
- Esteve Ràfols, M. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112.
- Mancuso, P. The role of adipokines in chronic inflammation. Immunol. Targets Ther. 2016, 5, 47.
- Sun, W.; Dong, H.; Balaz, M.; Slyper, M.; Drokhlyansky, E.; Colleluori, G.; Giordano, A.; Kovanicova, Z.; Stefanicka, P.; Balazova, L.; et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 2020, 587, 98–102.
- Vidal, H. Gene expression in visceral and subcutaneous adipose tissues. Ann. Med. 2001, 33, 547–555.
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and Subcutaneous Adipose Tissues of Obese Subjects Release Interleukin-6: Depot Difference and Regulation by Glucocorticoid 1. J. Clin. Endocrinol. Metab. 1998, 83, 847–850.
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261.
- Zhang, Y.; Huo, Y.; He, W.; Liu, S.; Li, H.; Li, L. Visfatin is regulated by interleukin-6 and affected by the PPAR-γ pathway in BeWo cells. Mol. Med. Rep. 2019, 19, 400–406.
- Klöting, N.; Graham, T.E.; Berndt, J.; Kralisch, S.; Kovacs, P.; Wason, C.J.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M.; et al. Serum Retinol-Binding Protein Is More Highly Expressed in Visceral than in Subcutaneous Adipose Tissue and Is a Marker of Intra-abdominal Fat Mass. Cell Metab. 2007, 6, 79–87.
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Chatterjee Bhowmick, D.; Murano, I.; Cohen, P.; et al. Adipsin Is an Adipokine that Improves β Cell Function in Diabetes. Cell 2014, 158, 41–53.
- Auguet, T.; Quintero, Y.; Terra, X.; Martínez, S.; Lucas, A.; Pellitero, S.; Aguilar, C.; Hernández, M.; Del Castillo, D.; Richart, C. Upregulation of Lipocalin 2 in Adipose Tissues of Severely Obese Women: Positive Relationship With Proinflammatory Cytokines. Obesity 2011, 19, 2295–2300.
- Winkler, G.; Kiss, S.; Keszthelyi, L.; Sápi, Z.; Ory, I.; Salamon, F.; Kovács, M.; Vargha, P.; Szekeres, O.; Speer, G.; et al. Expression of tumor necrosis factor (TNF)-alpha protein in the subcutaneous and visceral adipose tissue in correlation with adipocyte cell volume, serum TNF-alpha, soluble serum TNF-receptor-2 concentrations and C-peptide level. Eur. J. Endocrinol. 2003, 149, 129–135.
- Lee, K.Y.; Sharma, R.; Gase, G.; Ussar, S.; Li, Y.; Welch, L.; Berryman, D.E.; Kispert, A.; Blüher, M.; Kahn, C.R. Tbx15 Defines a Glycolytic Subpopulation and White Adipocyte Heterogeneity. Diabetes 2017, 66, 2822–2829.
- Lee, K.Y.; Luong, Q.; Sharma, R.; Dreyfuss, J.M.; Ussar, S.; Kahn, C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 2019, 38, e99291.
- Ramirez, A.K.; Dankel, S.N.; Rastegarpanah, B.; Cai, W.; Xue, R.; Crovella, M.; Tseng, Y.; Kahn, C.R.; Kasif, S. Single-cell transcriptional networks in differentiating preadipocytes suggest drivers associated with tissue heterogeneity. Nat. Commun. 2020, 11, 2117.
- Preisner, F.; Leimer, U.; Sandmann, S.; Zoernig, I.; Germann, G.; Koellensperger, E. Impact of Human Adipose Tissue-Derived Stem Cells on Malignant Melanoma Cells in An In Vitro Co-culture Model. Stem Cell Rev. Rep. 2018, 14, 125–140.
- Salha, S.; Gehmert, S.; Brébant, V.; Anker, A.; Loibl, M.; Prantl, L.; Gehmert, S. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clin. Hemorheol. Microcirc. 2019, 70, 543–551.
- Li, W.; Xu, H.; Qian, C. c-Kit-Positive Adipose Tissue-Derived Mesenchymal Stem Cells Promote the Growth and Angiogenesis of Breast Cancer. Biomed Res. Int. 2017, 2017, 7407168.
- Zimmerlin, L.; Park, T.S.; Zambidis, E.T.; Donnenberg, V.S.; Donnenberg, A.D. Mesenchymal stem cell secretome and regenerative therapy after cancer. Biochimie 2013, 95, 2235–2245.
- Guo, W.; Wong, S.; Xie, W.; Lei, T.; Luo, Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2007, 293, 576–586.
- Renovato-Martins, M.; Moreira-Nunes, C.; Atella, G.C.; Barja-Fidalgo, C.; Moraes, J.A. de Obese Adipose Tissue Secretion Induces Inflammation in Preadipocytes: Role of Toll-Like Receptor-4. Nutrients 2020, 12, 2828.
- La Cava, A.; Matarese, G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004, 4, 371–379.
- Lu, B.; Rutledge, B.J.; Gu, L.; Fiorillo, J.; Lukacs, N.W.; Kunkel, S.L.; North, R.; Gerard, C.; Rollins, B.J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J. Exp. Med. 1998, 187, 601–608.
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270.
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505.
- Rouault, C.; Marcelin, G.; Adriouch, S.; Rose, C.; Genser, L.; Ambrosini, M.; Bichet, J.-C.; Zhang, Y.; Marquet, F.; Aron-Wisnewsky, J.; et al. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia 2021, 64, 240–254.
- Miller, M.C.; Mayo, K.H. Chemokines from a Structural Perspective. Int. J. Mol. Sci. 2017, 18, 2088.
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928.
- Clore, G.M.; Gronenborn, A.M. Three-dimensional structures of α and β chemokines. FASEB J. 1995, 9, 57–62.
- Alexander, J.M.; Nelson, C.A.; Van Berkel, V.; Lau, E.K.; Studts, J.M.; Brett, T.J.; Speck, S.H.; Handel, T.M.; Virgin, H.W.; Fremont, D.H. Structural basis of chemokine sequestration by a herpesvirus decoy receptor. Cell 2002, 111, 343–356.
- Cochran, B.H.; Reffel, A.C.; Stiles, C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell 1983, 33, 939–947.
- Rollins, B.J.; Morrison, E.D.; Stiles, C.D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc. Natl. Acad. Sci. USA 1988, 85, 3738–3742.
- Mehrabian, M.; Sparkes, R.S.; Mohandas, T.; Fogelman, A.M.; Lusis, A.J. Localization of monocyte chemotactic protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1. Genomics 1991, 9, 200–203.
- Barna, B.P.; Pettay, J.; Barnett, G.H.; Zhou, P.; Iwasaki, K.; Estes, M.L. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 1994, 50, 101–107.
- Cushing, S.D.; Berliner, J.A.; Valente, A.J.; Territo, M.C.; Navab, M.; Parhami, F.; Gerrity, R.; Schwartz, C.J.; Fogelman, A.M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc. Natl. Acad. Sci. USA 1990, 87, 5134–5138.
- Brown, Z.; Strieter, R.M.; Neild, G.H.; Thompson, R.C.; Kunkel, S.L.; Westwick, J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992, 42, 95–101.
- Standiford, T.J.; Kunkel, S.L.; Phan, S.H.; Rollins, B.J.; Strieter, R.M. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 1991, 266, 9912–9918.
- Yoshimura, T.; Yuhki, N.; Moore, S.K.; Appella, E.; Lerman, M.I.; Leonard, E.J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989, 244, 487–493.
- Yoshimura, T.; Robinson, E.A.; Tanaka, S.; Appella, E.; Kuratsu, J.I.; Leonard, E.J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J. Exp. Med. 1989, 169, 1449–1459.
- Gerhardt, C.C.; Romero, I.A.; Cancello, R.; Camoin, L.; Strosberg, A.D. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol. Cell. Endocrinol. 2001, 175, 81–92.
- Gschwandtner, M.; Derler, R.; Midwood, K.S. More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis. Front. Immunol. 2019, 10, 2759.
- Tsaur, I.; Noack, A.; Makarevic, J.; Oppermann, E.; Waaga-Gasser, A.M.; Gasser, M.; Borgmann, H.; Huesch, T.; Gust, K.M.; Reiter, M.; et al. CCL2 Chemokine as a Potential Biomarker for Prostate Cancer: A Pilot Study. Cancer Res. Treat. 2014, 47, 306–312.
- Lubowicka, E.; Przylipiak, A.; Zajkowska, M.; Piskór, B.M.; Malinowski, P.; Fiedorowicz, W.; Ławicki, S. Plasma Chemokine CCL2 and Its Receptor CCR2 Concentrations as Diagnostic Biomarkers for Breast Cancer Patients. Biomed Res. Int. 2018, 2018, 2124390.
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419.
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266.
- Aljada, A.; Ghanim, H.; Saadeh, R.; Dandona, P. Insulin Inhibits NFκB and MCP-1 Expression in Human Aortic Endothelial Cells. J. Clin. Endocrinol. Metab. 2001, 86, 450–453.
- Nakatsumi, H.; Matsumoto, M.; Nakayama, K.I. Noncanonical Pathway for Regulation of CCL2 Expression by an mTORC1-FOXK1 Axis Promotes Recruitment of Tumor-Associated Macrophages. Cell Rep. 2017, 21, 2471–2486.
- Bose, S.; Cho, J. Role of chemokine CCL2 and its receptor CCR2 in neurodegenerative diseases. Arch. Pharm. Res. 2013, 36, 1039–1050.
- Callewaere, C.; Banisadr, G.; Rostène, W.; Parsadaniantz, S.M. Chemokines and chemokine receptors in the brain: Implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007, 38, 355–363.
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interf. Cytokine Res. 2009, 29, 313–325.
- Oettgen, H.; Broide, D.H. Introduction to mechanisms of allergic disease. In Allergy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–32.
- Charo, I.F.; Myers, S.J.; Herman, A.; Franci, C.; Connolly, A.J.; Coughlin, S.R. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc. Natl. Acad. Sci. USA 1994, 91, 2752–2756.
- Bartoli, C.; Civatte, M.; Pellissier, J.; Figarella-Branger, D. CCR2A and CCR2B, the two isoforms of the monocytes chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol. 2001, 102, 385–392.
- Jen, C.H.; Leary, J.A. A competitive binding study of chemokine, sulfated receptor, and glycosaminoglycan interactions by nano-electrospray ionization mass spectrometry. Anal. Biochem. 2010, 407, 134–140.
- Blanc, R.S.; Kallenbach, J.G.; Bachman, J.F.; Mitchell, A.; Paris, N.D.; Chakkalakal, J.V. Inhibition of inflammatory CCR2 signaling promotes aged muscle regeneration and strength recovery after injury. Nat. Commun. 2020, 11, 4167.
- Sarafi, M.N.; Garcia-Zepeda, E.A.; MacLean, J.A.; Charo, I.F.; Luster, A.D. Murine Monocyte Chemoattractant Protein (MCP)-5: A Novel CC Chemokine That Is a Structural and Functional Homologue of Human MCP-1. J. Exp. Med. 1997, 185, 99–110.
- Nomiyama, H.; Hieshima, K.; Nakayama, T.; Sakaguchi, T.; Fujisawa, R.; Tanase, S.; Nishiura, H.; Matsuno, K.; Takamori, H.; Tabira, Y.; et al. Human CC chemokine liver-expressed chemokine/CCL16 is a functional ligand for CCR1, CCR2 and CCR5, and constitutively expressed by hepatocytes. Int. Immunol. 2001, 13, 1021–1029.
- Sozzani, S.; Zhou, D.; Locati, M.; Rieppi, M.; Proost, P.; Magazin, M.; Vita, N.; van Damme, J.; Mantovani, A. Receptors and transduction pathways for monocyte chemotactic protein-2 and monocyte chemotactic protein-3. Similarities and differences with MCP-1. J. Immunol. 1994, 152, 3615–3622.
- Mellado, M.; Rodríguez-Frade, J.M.; Aragay, A.; del Real, G.; Martín, A.M.; Vila-Coro, A.J.; Serrano, A.; Mayor, F.; Martínez-A, C. The chemokine monocyte chemotactic protein 1 triggers Janus kinase 2 activation and tyrosine phosphorylation of the CCR2B receptor. J. Immunol. 1998, 161, 805–813.
- Cambien, B.; Pomeranz, M.; Millet, M.A.; Rossi, B.; Schmid-Alliana, A. Signal transduction involved in MCP-1-mediated monocytic transendothelial migration. Blood 2001, 97, 359–366.
- Wain, J.H.; Kirby, J.A.; Ali, S. Leucocyte chemotaxis: Examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by Monocyte Chemoattractant Proteins-1, -2, -3 and -4. Clin. Exp. Immunol. 2002, 127, 436–444.
- UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515.
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 receptor-mediated calcium signaling and its role in autophagy in cancer. Front. Oncol. 2017, 7, 1.
- Racioppi, L.; Noeldner, P.K.; Lin, F.; Arvai, S.; Means, A.R. Calcium/calmodulin-dependent protein kinase kinase 2 regulates macrophage-mediated inflammatory responses. J. Biol. Chem. 2012, 287, 11579–11591.
- McCoy, F.; Eckard, L.; Chen, S.; Nutt, L.K. Metabolic regulation of apoptosis via Ca+/Calmodulin Kinase II (CaMKII). BMC Proc. 2012, 6, P36.
- Sriraman, V.; Modi, S.R.; Bodenburg, Y.; Denner, L.A.; Urban, R.J. Identification of ERK and JNK as signaling mediators on protein kinase C activation in cultured granulosa cells. Mol. Cell. Endocrinol. 2008, 294, 52–60.
- Dawson, J.; Miltz, W.; Mir, A.K.; Wiessner, C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin. Ther. Targets 2003, 7, 35–48.
- Pessentheiner, A.R.; Ducasa, G.M.; Gordts, P.L.S.M. Proteoglycans in Obesity-Associated Metabolic Dysfunction and Meta-Inflammation. Front. Immunol. 2020, 11, 769.

