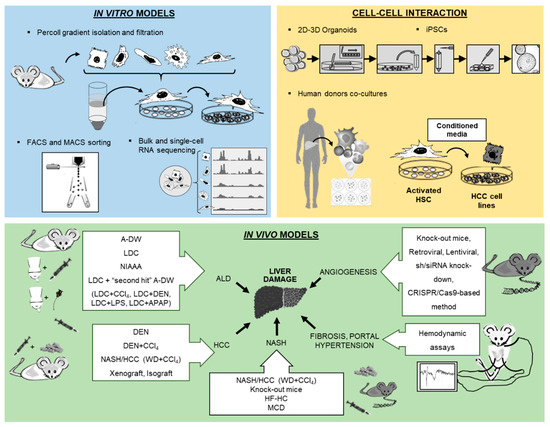
Molecular and cellular research modalities for the study of liver pathologies have been tremendously improved over the recent decades. Advanced technologies offer novel opportunities to establish cell isolation techniques with excellent purity, paving the path for 2D and 3D microscopy and high-throughput assays (e.g., bulk or single-cell RNA sequencing). The use of stem cell and organoid research will help to decipher the pathophysiology of liver diseases and the interaction between various parenchymal and non-parenchymal liver cells. Furthermore, sophisticated animal models of liver disease allow for the in vivo assessment of fibrogenesis, portal hypertension and hepatocellular carcinoma (HCC) and for the preclinical testing of therapeutic strategies. The purpose of this review is to portray in detail novel in vitro and in vivo methods for the study of liver cell biology that had been presented at the workshop of the 8th meeting of the European Club for Liver Cell Biology (ECLCB-8) in October of 2018 in Bonn, Germany.
Cell culture techniques are important tools for the study of pathogenesis and treatment of liver diseases. Each different cell type plays a specific role in the liver. The parenchymal cells, mainly hepatocytes, constitute 80% of the total liver volume and are responsible for the majority of liver functions. The nonparenchymal cells include hepatic stellate cells (HSCs), liver sinusoidal endothelial cells and resident macrophages such as Kupffer cells (KCs)
[1]. The connective tissue of the liver consists of blood vessels, nerves, lymphatic vessels, bile canaliculi and extracellular matrix [
2]. Inside the bile canaliculi, the epithelial cells, the so-called cholangiocytes, participate in the regulation of bile production and in the process of biliary repair [
3]. In recent years, biliary research and cholangiocytes gained much interest in the research of liver diseases. Although conventional procedures to isolate liver cells, such as in situ liver perfusion and collagenase digestion followed by density gradient isolation, are well established, novel methods have been introduced such as fluorescence-activated cell sorting (FACS) of HSCs using vitamin A autofluorescence. These approaches provide high purity of HSCs and are reproducible. In the coming years, the use of 2D microscopy, 3D microscopy, intravital microscopy, flow cytometry and cell isolation for subsequent functional experiments or expression analyses is expected to significantly increase characterization of hepatic cell populations and their interactions with circulating immune cells. For instance, these methods have been used to study the impact of hepatic macrophages and monocytes on steatosis, inflammation, hepatocellular injury, HSCs activation and angiogenesis [
4]. Two novel technologies from stem cell research, induced pluripotent stem cells (iPSCs) and liver organoids, helped to decipher the pathophysiology of cholangiopathies [
5]. Using in vitro models of primary human liver cells from different human donors, co-culture systems can simulate additional pathophysiological liver cell interactions, being complementary to animal models in preclinical analysis. Furthermore, development of animal models to experimentally mimic alcoholic liver disease (ALD), such as the liquid Lieber–DeCarli (LDC) diet, intragastric ethanol infusion and administration of carbon tetrachloride (CCl
4) combined with ethanol in drinking water, can help to understand critical pathophysiological steps of human ALD, such as inflammation and fibrosis during ALD progression [
6,
7]. Moreover, HCC models are receiving increased attention, not only for the fast xenograft model, but also for application of
N-nitrosodiethylamine (DEN) followed by repeated administration of CCl
4 () [
8], as well as the model of non-alcoholic steatohepatitis (NASH) combined with a HCC model described recently by using a Western diet (WD) combined with CCl
4 () [
7,
9]. Finally, the assessment of systemic, splanchnic and portal hemodynamics in animal models is now well accepted and crucial for the evaluation of drugs for end-stage liver disease. The purpose of this review is to present technological tools that will enhance our ability to isolate and purify the different cell populations and other strategies to study the full spectrum of liver disease ().
Figure 1. Overview of new methodologies using in vitro, cell–cell interaction and in vivo models for the study of liver pathology.
Table 1. Select findings of new methodology applications in in vitro organoids and vivo models.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21062027