Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Matthew Stoyek and Version 2 by Rita Xu.
The intracardiac nervous system (IcNS), sometimes referred to as the “little brain” of the heart, is involved in modulating aspects of cardiac physiology. The IcNS is composed of neuronal and non-neuronal compartments intrinsic to the heart and includes afferent, efferent, and intra- neurons, using sympathetic, parasympathetic, and non-adrenergic, non-cholinergic neural transmitters, and forming feedback loops with the central (brain and spine) and peripheral (paravertebral ganglia) nervous systems.
- neurocardiology
- sympathetic
- parasympathetic
- mammal
- zebrafish
1. Introduction
The presence of an intracardiac nervous system (IcNS) has intrigued scientists for more than 200 years, with the first published reference by Scarpa in the Tabulae Nevrologicae in 1794 [1]. In the early 20th century, Cannon [2] and Langley [3] provided more detailed descriptions of the structural, functional, and pharmacological properties of visceral organ control by the autonomic nervous system (ANS). Traditionally, the ANS is described as an efferent system that provides control over visceral organ function that is critical for maintaining homeostasis, including that of the cardiovascular system. The ANS is composed of two principal efferent divisions, the parasympathetic and sympathetic nervous systems. Classically, regarding their organ function control, these divisions have been viewed as having a reciprocal antagonistic relationship [2][3][2,3]. The IcNS is the final common pathway for the ANS in the heart [4][5][6][7][4,5,6,7], and facilitates rapid, reflex-driven beat-to-beat modulation of cardiac output to match changes in systemic demand. Sometimes referred to as the “little brain of the heart” [8], the ICNS accomplishes this by modulating various aspects of cardiac performance, including chronotropy, dromotropy, lusitropy, and inotropy [8][9][10][8,9,10].
In the traditional model of cardiac regulation, the central nervous system (CNS), encompassing the spinal cord, medulla, and higher brain centres, processes cardiovascular feedback, and relays signals to the heart via peripheral ANS nerves. In this configuration, the IcNS is simply a conduit for central inputs to the heart [8][11][8,11]. The view of autonomic control of the heart has since evolved, and is now considered as organised hierarchically into three functional ‘levels’ [8]. Level 1 is composed of neurons within the CNS, typically the medulla and spinal cord, which are controlled by higher brain centres [8][9][8,9]. Level 2 is composed of intrathoracic ganglia (e.g., stellate ganglia), sensory nerves, sympathetic and parasympathetic efferent neurons, and connects the CNS to the heart. Level 3 is composed of those neural elements which are intrinsic to the heart itself (i.e., the IcNS) [8]. Recent advances have shown that the IcNS is far more complex than the simple ‘neuronal-relay system’ of classical dogma. Work in the last two decades has revealed neurochemical complexity [12][13][12,13], neuronal distribution [8][12][14][15][8,12,14,15], capability for local information processing, and neural interactions not explained by our traditional understanding of the IcNS [8][16][8,16]. In addition to the neuronal components, the CNS and ANS (including the IcNS) also contain myelinating and Remak Schwann cells (SC), neural-associated fibroblasts and perycites, macrophages, and endothelial cells [17][18][17,18]. It is assumed that the cells of the non-neuronal compartment of the ANS support neuronal development, impulse conduction, metabolic maintenance, and regeneration, but the available information regarding these cell types is surprisingly limited.
There exist numerous established experimental models that can be used to study the IcNS. In choosing an appropriate model, the similarity of the relevant processes to those in humans determines their utility for expanding our mechanistic understanding of human physiology and pathophysiology.
1.1. Extrinsic Cardiac Regulation—The CNS and Intrathoracic Nervous System
The primary extrinsic control of cardiac activity is through the CNS, acting via extracardiac sympathetic and parasympathetic innervation of intracardiac neural circuits and cardiac tissues [19][20][21][19,20,21]. Preganglionic sympathetic neurons, typically originating from the reticular formation in the brainstem, including the ventrolateral medulla, project axons via the intermediolateral column of the spinal cord [8][22][8,22], exiting the spinal cord bilaterally and projecting to postganglionic intrathoracic neurons in the superior and middle cervical, cervicothoracic (stellate), and mediastinal ganglia [22][23][24][22,23,24], which project to the heart. Preganglionic parasympathetic neurons project axons from brainstem nuclei (e.g., dorsal vagal motor nuclei, insular cortex, nucleus ambiguus [8][9][8,9]) and travel via bilateral vagus nerves, and multiple cardiopulmonary branches, which form synapses with postganglionic parasympathetic neurons within cardiac ganglia [9]. Both the central (brain and spine) and intrathoracic (paravertebral ganglia) levels also receive information from afferent nerves originating from the heart, lungs, and vasculature [11].
It is worth noting that cardiac function may also be affected by neurohumoral agents (including peptides) produced in endocrine glands and released into the circulation [19][20][21][19,20,21]. Circulating epinephrine and norepinephrine released by the adrenal medulla (as well as dopamine, when converted to epinephrine or norepinephrine), will cause an increase in heart rate by a similar mechanism to neurotransmitters released locally by sympathetic neurons; however, the details of this are beyond the scope of this review.
1.2. Intrinsic Cardiac Regulation—The IcNS
It is now being shown that within the IcNS, in addition to intracardiac neurons, there also exist other cell types which play crucial roles in IcNS structure and function, including SC, small intensely fluorescent cells (SIF), and endoneurial fibroblasts (EF). Furthermore, it has been shown that the IcNS has the capability to exert reflex control of cardiac function, even when isolated from central and intrathoracic levels of the extracardiac ganglia and CNS [11][25][26][27][11,25,26,27].
1.2.1. Intracardiac Neurons
Intracardiac neurons derive primarily from neural crest cells (NCC) that migrate to the developing heart (~5th week in humans and ~E8.5–9.5 in mice [10][28][29][10,28,29]) giving rise to sympathetic, parasympathetic, and afferent sensory neurons [10][28][10,28]. Currently much of what is known of embryonic and fetal development of IcNS neurons and their subsequent postnatal maturation is derived from studies in mice. Sympathetic intracardiac neurons originate from trunk NCC that first migrate ventrally towards the dorsal aorta, and then rostrally and caudally, forming the paravertebral sympathetic chain [10]. NCC, which ultimately derive sympathetic components, have been shown to reach the dorsal aorta and outflow tract between E9.5-E10.5 [29]. By E13.5-E15.5, sympathetic axons begin to extend along coronary veins and penetrate the subepicardium, but it is not until E17.5 that these axons infiltrate the myocardium [30]. Recently, it has been reported that sympathetic maturation is largely completed by P7 [31]. Parasympathetic intracardiac neurons originate either from cardiac NCC, a subset of the vagal neural crest [10], where they migrate laterally to the somites before entering the heart, or from cells within the nodose placode (~E12.5 in mice [10][28][10,28]). NCC, which will contribute to parasympathetic components, have been observed at the venous pole of the heart at E12.5 [28]; however, development of parasympathetic innervation is believed to occur postnatally from P0.5-P21 [32]. Maturation of parasympathetic innervation is still ongoing at P30 and the exact time course to reach a terminal maturation is currently unknown [31]. Development of cardiac neurons can be considered a co-maturation system, in which signals from cardiac tissue regulate the growth, patterning, and transmission properties of intracardiac neurons, while reciprocal signalling from intracardiac neurons influences the maturation of cardiomyocytes (CM) [33].
Intracardiac neurons can be broadly characterised by: (i) their anatomical/topographical layout [34]; (ii) their neurochemical phenotype [8][35][36][8,35,36]; and (iii) their functional influence on the heart [35]. Analogous to the enteric nervous system of the digestive tract, intracardiac neurons are typically found within an aggregation known as a “ganglionated plexus” (GP) [9][14][34][9,14,34]. The exact locations of GP, as well as their patterns of innervation to the atria and ventricles, are highly species-dependent, though generally GP are supraventricular and found on the epicardium in intramural tissue or in epicardial fat pads [9][10][16][9,10,16]. In terms of neurochemical phenotypes, it is now known that within the IcNS there are parasympathetic, sympathetic, and non-noradrenergic, non-cholinergic transmitters and modulators, and along with extrinsic inputs, intracardiac circuits may be important for internal signal processing and intracardiac reflex control [8][36][8,36]. Functionally, there are two broad classes of intracardiac neurons. Type 1, also referred to as phasic or somatic neurons, are typically monopolar (with a single axonal projection) and fire only a single action potential (AP) during a sustained depolarising stimulus. Type 2 intracardiac neurons are multipolar and fire multiple AP during a sustained depolarising stimulus [10][37][10,37]. Type 2 neurons can be further sub-classified as slow after-hyperpolarisation neurons or pacemaker neurons, which are distinguished by slow or rapid repolarisation characteristics, respectively [10]. A third type of intracardiac neuron has also been demonstrated, referred to as an accommodating neuron, which fires multiple APs at a decreasing rate during sustained depolarisation [37].
Functional data have shown that intracardiac neurons receive inputs from extrinsic efferent parasympathetic preganglionic and sympathetic postganglionic neurons, local circuit neurons, and afferent neurons from locations across the heart [37][38][37,38]. As a result, even after acute [38] and chronic [25] isolation of the heart from the CNS (“decentralisation”), the IcNS remains responsive to changes in the cardiac environment. In addition, by simultaneous recording it has been demonstrated that parasympathetic (vagal) and sympathetic (cardiopulmonary) nerves can be co-activated [39][40][41][39,40,41], and that adrenergic-cholinergic interactions can augment activity of the IcNS in a manner that does not occur in the presence of only one input [37]. This may be less surprising when one considers that it has been shown that there are intracardiac neurons which respond weakly, or do not respond, to direct vagal stimulation simulating CNS input [8][25][8,25].
1.2.2. Intracardiac Schwann Cells (SC)
SC in the heart are neural crest derived glial cells that are necessary for nerve fasciculation in development [31]. While whole heart expression patterns are not currently known, electron [17] and fluorescent [31] imaging have described the presence of two distinct SC populations: (i) a small population of myelinating SC [42] and (ii) a prevalent population of non-myelinating cells, known as Remak SC, which are thought to play a role in development and regeneration [31][43][31,43]. An initial description of cardiac SC gene expression in homeostasis has been shown by single cell RNA sequencing [44], but the role of these cells in cardiac disease remains unexplored.
1.2.3. Small Intensely Fluorescent (SIF) Cells
SIF cells have long been known to exist within the CNS, and while they have been described in the ganglia of the heart [12], their presence remains incompletely understood. It is known that these small ‘neuron-like’ cells are catecholaminergic, with strong immunoreactivity for tyrosine hydroxylase (TH; the rate limiting enzyme in catecholamine synthesis [12][45][12,45]). While the presence of TH confirms a catecholaminergic phenotype, these cells lack immunoreactivity to dopamine beta-hydroxylase, the enzyme involved in the conversion of dopamine to norepinephrine. In some cases, they exhibit immunoreactivity to tryptophan hydroxylase, which is involved in the synthesis of serotonin (or 5-HT). Further to this, it has been reported that cholinergic synaptic terminals are frequently observed near SIF cells [12], and it has been proposed that SIF cells may have endocrine, chemoreceptive, or interneuronal roles [10]. Ultimately, the origins and role of SIF cells within the IcNS remains to be explored.
1.2.4. Intracardiac Endoneurial Fibroblasts (EF)
While they have been known in other organs for decades, EF had not been described in the heart until recently [31]. Aggregations of EF around thick nerves of the atria, outflow tract, and epicardium were found to be of neural crest origin, while EF that supported thinner intraventricular nerves are probably derived from epi- or endocardial precursors [31]. It is assumed that EF play a crucial role in nerve extracellular matrix homeostasis, but due to the lack of experimental tools such as cell-specific markers, there is only minimal data available regarding this cell type in the heart.
1.3. Therapeutic Potential of IcNS Targets
In recent years our understanding of the specifics of ANS control of the heart has greatly expanded and the ANS is increasingly being implicated in cardiovascular disease [8][9][46][47][8,9,46,47]. However, investigation of the specific functional roles of subpopulations of intracardiac neurons has been hampered by a lack of knowledge regarding the functional network interactions of the neural and non-neural compartments of the IcNS. Multiple levels of the CNS and IcNS, comprising several integrated feedback loops, are involved in the regulation of ANS function and may be disturbed in pathology. These include intracardiac cardio-cardiac reflexes and intrinsic cardiac nerve activity that alter nerve transmission within the myocardium, intrathoracic reflexes and feedback mechanisms that modify sympathetic ganglionic efferent transmission, and spinal, lower brainstem, and higher brain centre regulation that modulates autonomic outflow [48][49][50][48,49,50].
Chronic alterations in sympathetic/parasympathetic balance are a well-established contributor to many cardiovascular diseases, being strongly linked to clinical outcome and prognosis [48][51][48,51], and emerging clinical data highlight a role for intracardiac neurons in ANS imbalance and the resulting disease progression [51].
It has also been shown that the IcNS may play a role in genetic disorders (e.g., Brugada and Long QT syndrome), neurodegenerative conditions (e.g., epilepsy, dysautonomia), myocardial injury, acquired arrhythmias (such as atrial fibrillation), and contractile dysfunction [52]. Furthermore, associated neural remodelling [53] can alter ion channel function and signalling cascades, and thus impact cardiac function [33][52][33,52]. Currently, most therapeutic interventions in pathological states such as heart failure and arrhythmia are pharmacologic and impact the entire neurohormonal axis [9]. More targeted neuromodulatory treatments are currently being explored in pre-clinical and clinical studies, including vagal nerve stimulation, cardiac sympathetic denervation, renal denervation, spinal cord stimulation, baroreflex activation, neurotoxin injection, and tragus stimulation [47][54][47,54].
In the setting of heart transplantation, where the donor heart has been decentralised, reinnervation may occur in an inconsistent and incomplete manner [55]. This decentralisation has been related to a depletion in circulating catecholamines, reduced exercise capacity, increased resting heart rate, and disturbed blood pressure regulation [55][56][55,56]. Thus, it is clear that a comprehensive understanding of how the nervous system controls the heart, and what can go wrong with this control system, is a key factor in developing successful new neuronally-based cardiac therapies [54][57][54,57].
2. Mammalian Models of the IcNS
Mammalian models, including large animals (e.g., pigs, sheep, dogs), smaller animals (e.g., rabbits), and most commonly rodents (e.g., mice, rats, and guinea pigs) have long been the mainstays for experimental neurocardiology.
2.1. Anatomy of the IcNS
GP from small mammals appear to be discretely located (although their arrangement is species-dependent), but are more diffusely distributed in larger mammals. Based on neuronal inputs and intracardiac projections to effector cells, GP have been generalised into five to seven regions (Figure 1): (i) right dorsal atrial; (ii) ventral right atrial; (iii) left dorsal; (iv) ventral left atrial; (v) middle dorsal; (vi) right coronary; and (vii) left coronary [9]. GP in all of these regions have been demonstrated in the rabbit [58], dog [14], sheep [59], and human [15][18][15,18]. Intrinsic cardiac nerves extend epicardially from GP to innervate the atria, interatrial septum, and the ventricles [18][59][60][18,59,60] (Figure 2A,B). Two subplexus routes extend from the arterial region of the hilum between the pulmonary trunk and the aorta to effector sites on the left and right ventricles. A further five sub-GP are found in the venous region of the heart, including: (i) dorsal, at the right caudal vein or the superior vena cava; (ii) middle dorsal, at the pulmonary veins; (iii) left dorsal; (iv) ventral left atrial; and (v) right ventral. The defined routes of these GP are comparatively similar amongst mammals [9]. A complete mapping of the GP and their projections can be found in the review by Wake and Brack [35].
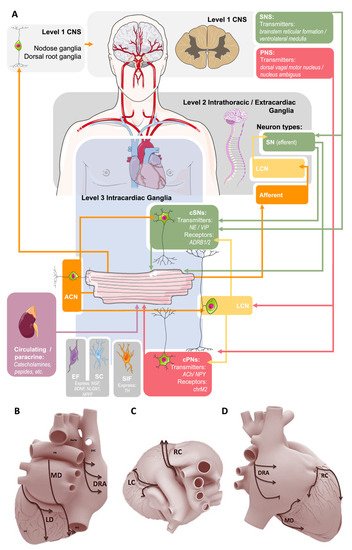
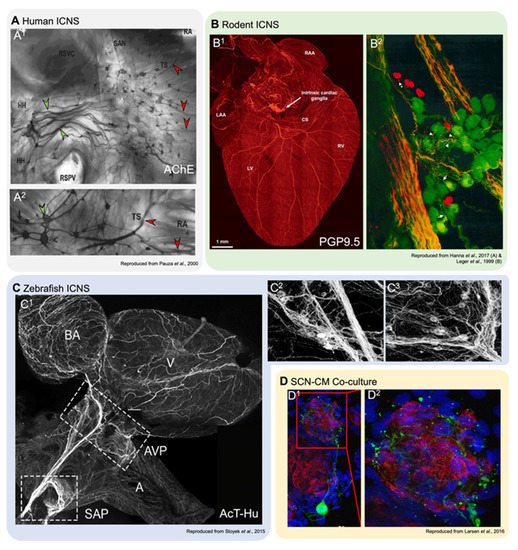

Figure 1. Summary of the current conceptual model of autonomic control of the heart. (A): Sympathetic innervation (SN, green) originates from the central (Level 1) and intrathoracic (Level 2) levels and projects to cardiac sympathetic neurons (cSNs) within the intracardiac nervous system (IcNS, Level 3). Parasympathetic (PNS) innervation (red) comes from the central level via the vagus nerve to cardiac parasympathetic (cPNs) and local circuit (LCN) neurons within the IcNS. Sensory afferent neurons (orange) project to nodose and dorsal root ganglia, and afferent cardiac neurons (ACN) project to SCNs and LCNs. Arrowheads indicate direction of nerve conduction. At the level of the IcNS there are also small, intensely fluorescent (SIF) cells, Schwann cells (SC), and endoneurial fibroblasts (EF). Parts of the illustration were adapted from Servier SMART under a CC BY 3.0 license. (B): A proposed scheme of innervation of the mammalian heart by the cardiac ganglionated plexuses in dorsal (B), right lateral (C), and superior (D) views. Ganglia: DRA, dorsal right atrial; LC, left coronary; LD, left dorsal; MD, middle dorsal; RC, right coronary. Reproduced with permission from [22].

Figure 2. Innervation of cardiac tissues in human and in experimental models. (A1,2): Acetylcholinesterase staining of right atrial human atrial innervation. Thicker nerves situated over the ganglia (green arrowheads), which are stained for AChE, are paler than the smaller ganglia (red arrowheads; reproduced with permission from [15]). HH, heart hilum; RA, right atrium; RSPV, right superior pulmonary vein; RSVC, root of the superior vena cava; SAN, sinoatrial node; TS, terminal sulcus. (B1,2): Dorsal view of a mouse heart (B1) stained with pan-neuronal marker PGP9.5 (protein gene product 9.5; reproduced with permission from [50]), and a detailed view of a left atrial ganglia from a guinea pig (B2) stained with PGP9.5 (green) and TH (red), showing neurons and SIF cells (reproduced with permission from [12]). CS, coronary sinus; LAA, left atrial appendage; LV, left ventricle; RAA, right atrial appendage; RV, right ventricle. (C1−3): Overview of innervation in the zebrafish heart (C1) and detail-views of left (C2) and right (C3) vagal ganglia, stained with pan-neuronal acetylated tubulin and human neuronal protein C/D (reproduced with permission from [61][64]). A, atrium; AVP, atrioventricular plexus; BA, bulbous arteriosus; SAP, sinoatrial plexus; V, ventricle. (D1,2): Co-cultures of rat ventricular myocytes and sympathetic stellate neurons. Alpha actinin marks myocytes (red), TH (green) is a sympathetic neuron marker, and nuclear marker DAPI (blue; reproduced with permission from [51]).
In contrast to our understanding of cardiac innervation of the atria, that of the ventricles remains less well understood and underappreciated. Mammalian ventricles were historically believed to be devoid of ganglia and innervation from the IcNS until ventricular ganglia were found in the dog [62][61] and human [38] at the coronary groove and around the conus arteriosus (CA). These findings have since been replicated in humans [18], sheep [59], dog [14], mice [63][64][62,63], and rabbit [58][60][58,60]. A more detailed investigation of the extremely rich intraventricular innervation has been performed recently using high-resolution fluorescence microscopy, which suggests there are direct neuronal effects on CM through neurotransmitter release from nearby varicosities [35].
In terms of neurotransmitters, it has been shown that sympathetic cardiac neurons generally contain norepinephrine, and in some instances the co-transmitters ATP, neuropeptide-Y (NPY), and galanin [33]. Parasympathetic cardiac neurons contain acetylcholine and co-transmitters such as natriuretic peptide Y, somatostatin, vasoactive intestinal polypetide, and endogenous opioids [33]. The presence of small catecholaminergic SIF cells associated with cholinergic innervation has also been described within the IcNS of guinea pig, which could modulate cardiac neural activity [12]. Moreover, a recent study described neuronal somata that exhibit co-localised immunoreactivity for choline acetyltransferase (ChAT, a rate limiting enzyme in the synthesis of acetylcholine) and TH within ganglia of the mouse heart [64][63], suggesting that parasympathetic and sympathetic transmitters may occur within the same intracardiac neurons [33].
2.2. Function of the IcNS
Acute [38] or chronic [25][27][25,27] decentralisation of the heart from the CNS has shown that intracardiac neurons in the mammalian heart remain viable and responsive to changes in the cardiac milieu. A complex and integrative view of the autonomic control of cardiac function has thus emerged, supplanting beliefs that individual GP solely innervate regions adjacent to the GP. It is now well-documented that GP can innervate and alter the function of both proximal and distant regions via intra- and inter-ganglionic communication [65][66][67][65,66,67]. The coordination of neuronal inputs and outputs within the IcNS depends on the nature of the afferent nerve supply, the efferent neuronal inputs received from the central/peripheral nervous systems through the sympathetic and parasympathetic nervous systems, and intrinsic connections via local circuit neurons within the IcNS.
An example of this is found in the posterior atrial GP, which receives inputs from sympathetic fibers from the right stellate ganglion, while parasympathetic intracardiac neurons from that GP project to the SAN and exert a negative chronotropic effect [68][69][68,69]. Thus, while it may be tempting to imagine that a specific IcNS GP (e.g., the right atrial GP) could be ablated to affect a particular cardiac region (e.g., the SAN), pleiotropic effects of GP result in off-target effects [9][70][71][9,70,71]. In fact, it has been shown that direct electrical stimulation of the cardiac vagal nerves (to simulate CNS input) results in weak evoked potentials in some intracardiac neurons in the beating mammalian heart, but these fail to fire action potentials (i.e., subthreshold stimulation) [8][37][72][73][74][75][76][8,37,72,73,74,75,76]. From those findings it is hypothesised that these ‘orphaned’ intracardiac neurons, which lack extrinsic input, may be acting as local circuit neurons. As more refined methodologies develop, researchers are now utilising molecular analyses to better characterise differences in intracardiac neuron populations. For instance, a recent study provided the first report of single cell RNA sequencing and functional analysis of stellate ganglia neurons, validated against human stellate ganglia neurons, in an effort to better characterise neurons innervating the heart [77].
2.3. Considerations for Investigations Using Mammalian Models
A major issue requiring further clarification for understanding the control of cardiac function by the IcNS is the distribution and connectivity of intracardiac neurons. The IcNS is estimated to comprise 43,000–94,000 neurons in the human heart, ~80,000 in dog, ~12,000 in pig, ~2200 in rabbit and guinea pig, ~6500 in rat, and ~1000 in mouse [35]. In the mammalian heart, intracardiac neuronal somata are widely distributed in ganglia throughout both atria [12][13][14][17][58][64][12,13,14,17,58,63], but with few exceptions (i.e., dog [78] and guinea pig [13]) the projection patterns of subpopulations of intracardiac neurons to specific targets remain largely unknown.
In mammalian hearts, a major difficulty in studying the role of specific components of the IcNS is their visualisation (Figure 2A,B) and manipulation. The IcNS is deeply embedded in, and distributed throughout, the walls of both the atria and ventricles, and thus largely inaccessible for integrative studies. One solution to this problem is the use of ‘reduced’ experimental preparations representing a subset of the IcNS, such as isolated tissues containing intracardiac neurons in GP, to study properties of control circuits in vitro [25][27][25,27]. This approach, however, eliminates a majority of intracardiac neuronal connections, so only limited conclusions may be drawn about the roles of localised circuitry in controlling overall cardiac function.
For structural microscopy studies, recently developed techniques such as optical clearing protocols, have been successfully applied to hearts of smaller mammalian models [79][80][81][79,80,81]. Even with small hearts, this method can take weeks to process, requires highly specialised imaging equipment, and involves time-consuming and technically challenging sequential imaging and reconstruction approaches to provide a view of cardiac innervation on an organ level. Most importantly, as it is conducted on fixed tissue, clearing-based imaging yields structural information only.
