Guided tissue regeneration (GTR) and guided bone regeneration (GBR) became common procedures in the corrective phase of periodontal treatment. In order to obtain good quality tissue neo-formation, most techniques require the use of a membrane that will act as a barrier, having as a main purpose the blocking of cell invasion from the gingival epithelium and connective tissue into the newly formed bone structure. Different techniques and materials have been developed, aiming to obtain the perfect barrier membrane. The membranes can be divided according to the biodegradability of the base material into absorbable membranes and non-absorbable membranes. The use of absorbable membranes is extremely widespread due to their advantages, but in clinical situations of significant tissue loss, the use of non-absorbable membranes is often still preferred.
- guided tissue regeneration
- guided bone regeneration
- barrier membranes
- allergology
1. Introduction
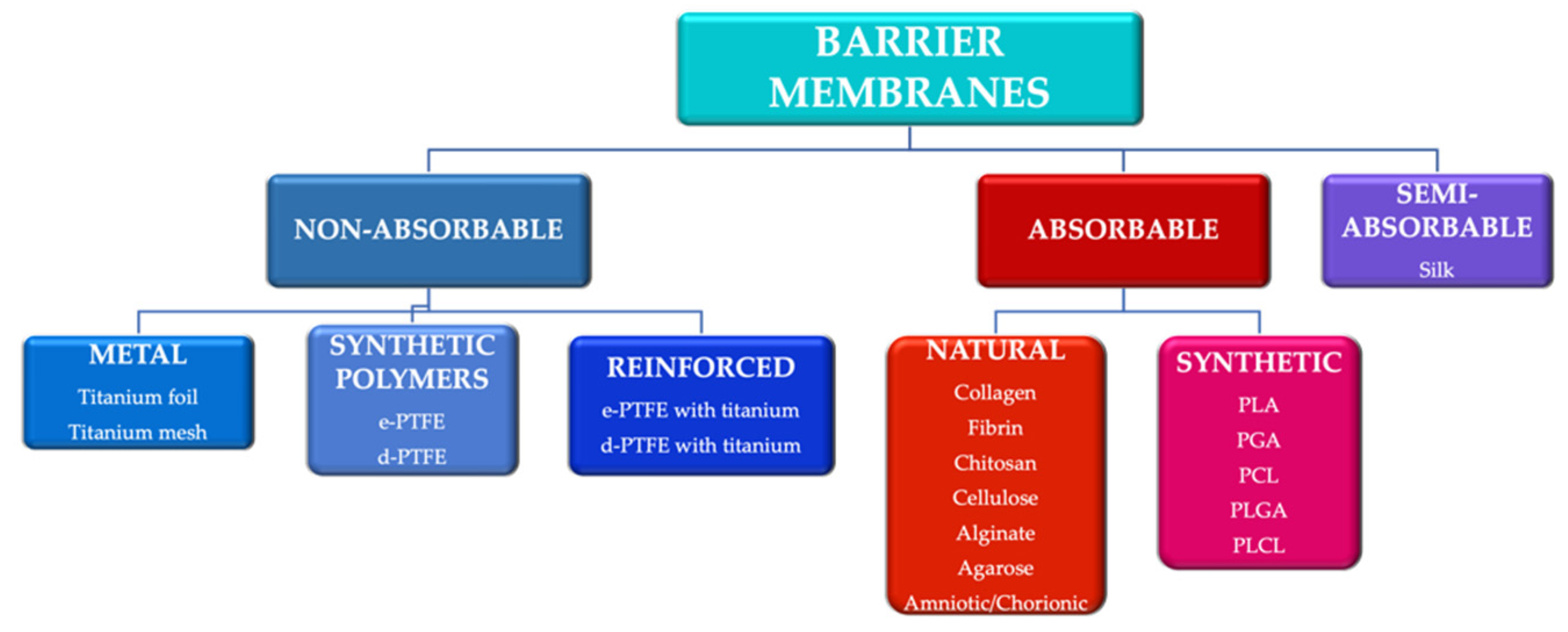
2. Trends in the Development of Barrier Membranes
2.1. Amniotic and Chorionic Membranes
2.2. Barrier Membranes from PRF
2.3. 3D Printed Membranes
3. Allergological Considerations
References
- Cheng, X.; Yang, F. More than just a barrier-challenges in the development of guided bone regeneration membranes. Matter 2019, 1, 550–644.
- Trombelli, L.; Heitz-Mayfield, L.; Needleman, I.; Moles, D.; Scabbia, A. A systematic review of graft materials and biological agents for periodontal intraosseous defects. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 117–135.
- Murphy, K.G.; Gunsolley, J.C. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann. Periodontol. 2003, 8, 266–302.
- Needleman, I.G.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R.J. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006, 19, CD001724.
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant. Dent. 2006, 15, 8–17.
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260.
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65.
- Caballe-Serano, J.; Abdeslam-Mohammed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernandez-Alfaro, F.; Miron, R. Adsorption and release kinetics of growth factors on barrier membranes for guided tissue/bone regeneration: A systematic review. Arch. Oral Biol. 2019, 100, 57–68.
- Sanz, M.; Dahlin, C.; Apatzidou, D.; Artzi, Z.; Bozic, D.; Calciolari, E.; De Bruyn, H.; Dommisch, H.; Donos, N.; Eickholz, P.; et al. Biomaterials and regenerative technologies used in bone regeneration in the craniomaxillofacial region: Consensus report of group 2 of the 15th European Workshop on Periodontology on Bone Regeneration. J. Clin. Periodontol. 2019, 46, 82–91.
- Tayebi, L.; Rasoulianboroujeni, M.; Moharamzadeh, K.; Almela, T.K.D.; Cui, Z.; Ye, H. 3D-printed membrane for guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 84, 148–158.
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81.
- Sasaki, J.I.; Abe, G.L.; Aonan, L.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63.
- Kaushal, S.; Kumar, A.; Khan, M.A.; Lal, N. Comparative study of nonabsorbable and absorbable barrier membranes in periodontal osseous defects by guided tissue regeneration. J. Oral Biol. Craniofac. Res. 2016, 6, 111–117.
- Lee, H.S.; Byun, S.H.; Cho, S.W.; Yang, B.E. Past, present, and future of regeneration therapy in oral and periodontal tissue: A review. Appl. Sci. 2019, 9, 1046.
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.-C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387.
- Shankar, P.; Kumar, A.; Kumari, C.B.N.; Mahendra, J.; Ambalavanan, N. Amnion and chorion membrane in periodontal regeneration. Ann. Rom. Soc. Cell Biol. 2020, 24, 435–441.
- Riau, A.K.; Beuerman, R.W.; Lim, L.S.; Mehta, J.S. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials 2010, 31, 216–225.
- Rodríguez-Ares, M.T.; López-Valladares, M.J.; Touriño, R.; Vieites, B.; Gude, F.; Silva, M.T.; Couceiro, J. Effects of lyophilization on human amniotic membrane. Acta Ophthalmol. 2009, 87, 396–403.
- Laurent, R.; Nallet, A.; Obert, L.; Nicod, L.; Gindraux, F. Storage and qualification of viable intact human amniotic graft and technology transfer to a tissue bank. Cell Tissue Bank. 2014, 15, 267–275.
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204.
- Shortt, A.J.; Secker, G.A.; Lomas, R.J.; Wilshaw, S.-P.; Kearney, J.N.; Tuft, S.J.; Daniels, J.T. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials 2009, 30, 1056–1065.
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458.
- Takashima, S.; Yasuo, M.; Sanzen, N.; Sekiguchi, K.; Okabe, M.; Yoshida, T.; Toda, A.; Nikaido, T. Characterization of laminin isoforms in human amnion. Tissue Cell 2008, 40, 75–81.
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater. 2008, 15, 88–99.
- Lei, J.; Priddy, L.B.; Lim, J.J.; Koob, T.J. Dehydrated human amnion/chorion membrane (dHACM) allografts as a therapy for orthopedic tissue repair. Tech. Orthop. 2017, 32, 149–157.
- Nishihara, S.; Someya, A.; Yonemoto, H.; Ota, A.; Itoh, S.; Nagaoka, I.; Takeda, S. Evaluation of the expression and enzyme activity of matrix metalloproteinase-7 in fetal membranes during premature rupture of membranes at term in humans. Reprod. Sci. 2008, 15, 156–165.
- Chen, E.; Tofe, A. A literature review of the safety and biocompatibility of amnion tissue. J. Implant. Adv. Clin. Dent. 2010, 2, 67–75.
- Ben Ali, L.M.S.; Mostafa Elazab, S. Amniotic membrane as a biodegradable barrier in technique of guided tissue regeneration in advanced periodontal disease in dogs: Histopathological assessment. Tissue Reg. Stem. Cell 2020, 2020, 1–16.
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical efficacy of amniotic membrane with biphasic calcium phosphate in guided tissue regeneration of intrabony defects- a randomized controlled clinical trial. Biomater. Res. 2015, 25, 15.
- Holtzclaw, D.J.; Toscano, N.J. Amnion–chorion allograft barrier used for guided tissue regeneration treatment of periodontal intrabony defects: A retrospective observational report. Clin. Adv. Periodontics 2013, 3, 131–137.
- Ríos, L.K.; Espinoza, C.V.; Alarcón, M.; Huamaní, J.O. Bone density of defects treated with lyophilised amniotic membrane versus collagen membrane: A tomographic and histomorfogenic study in rabbit’s femur. J. Oral Res. 2014, 3, 143–149.
- Sharma, A.; Yadav, K. Amniotic membrane—A Novel material for the root coverage: A case series. J. Indian Soc. Periodontol. 2015, 19, 444–448.
- Shetty, S.S.; Chatterjee, A.; Bose, S. Bilateral multiple recession coverage with platelet-rich fibrin in comparison with amniotic membrane. J. Indian Soc. Periodontol. 2014, 18, 102–106.
- Tseng, S.C.G. Grafts Made from Amniotic Membrane; Methods of Separating, Preserving and Using Such Grafts in Surgeries. U.S. Patent US6326019B1, 4 December 2001.
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for guided bone regeneration: A road from bench to bedside. Adv. Healthc. Mater. 2020, 9, 2000707.
- Miron, R.J.; Pikos, M.A. PRF as a barrier membrane in guided bone regeneration. Dent. Today 2017, 216, 36.
- Miron, R.J.; Bosshardt, D.D. OsteoMacs: Key players around bone biomaterials. Biomaterials 2016, 82, 1–19.
- Giannini, S.; Cielo, A.; Bonanome, L.; Rastelli, C.; Derla, C.; Corpaci, F.; Falisi, G. Comparison between PRP, PRGF and PRF: Lights and shadows in three similar but different protocols. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 927–930.
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360.
- Anilkumar, K.; Geetha, A.; Umasudhakar, T.R.; Vijayalakshmi, R.; Pameela, E. Platelet-rich-fibrin: A novel root coverage approach. J. Indian Soc. Periodontol. 2009, 13, 50–54.
- Jankovic, S.; Aleksic, Z.; Klokkevold, P.; Lekovic, V.; Dimitrijevic, B.; Kenney, E.B.; Camargo, P. Use of platelet-rich fibrin membrane following treatment of gingival recession: A randomized clinical trial. Int. J. Periodontics Restor. Dent. 2012, 32, e41–e50.
- Yeong, W.Y.; Chua, C.K.; Leong, K.F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652.
- Skardal, A.; Atala, A. Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746.
- Sears, N.A.; Seshadri, D.R.; Dhavalikar, P.S.; Cosgriff-Hernandez, E. A review of three-dimensional printing in tissue engineering. Tissue Eng. B Rev. 2016, 22, 298–310.
- Tamay, D.G.; Dursun Usal, T.; Alagoz, A.S.; Yucel, D.; Hasirci, N.; Hasirci, V. 3D and 4D printing of polymers for tissue engineering applications. Front. Bioeng. Biotechnol. 2019, 7, 164.
- Mobaraki, M.G.; Yazdanpanah, M.A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 2020, e00080.
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239.
- Bai, L.; Ji, P.; Li, X.; Gao, H.; Li, L.; Wang, C. Mechanical characterization of 3D-printed individualized Ti-mesh (membrane) for alveolar bone defects. J. Healthc. Eng. 2019, 2019, 4231872.
- Vanderburgh, J.; Sterling, J.A.; Guelcher, S.A. 3D printing of tissue engineered constructs for in vitro modeling of disease progression and drug screening. Ann. Biomed. Eng. 2017, 45, 164–179.
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001.
- Pacheco, K.A. Allergy to surgical implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85.
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; et al. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245.
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implant. Res. 2008, 19, 823–835.
- Demoly, P.; Michel, F.; Bousquet, J. Allergy, Principles and Practice, 5th ed.; Mosby-Year Book: St. Louis, MO, USA, 1998; pp. 430–439.
