1. Applications of P-GNS
P-GNS has been studied for its applications in electronic devices [
79,
82], energy storage devices [
77], and electrocatalysis [
75,
80], etc., due to the difference in the properties of the phosphorous and carbon atoms. These applications are generally divided into four categories, viz. lithium-ion batteries, solar cells, supercapacitors, and fuel cells. The applications of P-GNS synthesised using different approaches are listed in
Table 1.
Table 1. Different methods for synthesis of P-GNS.
| Synthesis Method |
Phosphorous Source |
Applications |
References |
| Pyrolysis method |
Phosphorous trichloride |
- |
[74] |
| Thermal annealing |
1-butyl-3-methylimidazolium hexafluorophosphate |
Electrocatalyst |
[75] |
| Chemical method |
Phosphoric acid + Polyphosphoric acid |
Flame retardant |
[76] |
| Templating method |
Phosphorous pentaoxide |
- |
[77] |
| Thermal annealing |
Phosphoric acid |
To retard oxidation of rGO |
[78] |
| Thermal decomposition |
Tri-n-octylphosphine + Tetradecylphosphonic +
Tri-n-butylphosphine
acid |
Supercapacitors |
[79] |
| Thermal annealing |
Phosphoric acid |
Fuel cells |
[80] |
| Chemical vapour discharge |
triphenylphosphine |
- |
[81] |
| Electrochemical erosion method |
Phosphoric acid |
Supercapacitor |
[82] |
| Chemical method |
Phosphoric acid + Polyphosphoric acid |
Supercapacitor |
[83] |
1.1. Fuel Cells
Fuel cells are used to convert the energy from a chemical nature to an electrical nature. Hydrogen gas is fed into the anodic compartment where it is oxidised and the oxygen gas is fed into the cathodic compartment where it is reduced. The ORR is the rate-determining step that occurs in the cathodic compartment [
111]. ORR can occur in the basic medium via two different routes: (i) either by a four-electron mechanism, or (ii) by two-electron pathway. Each electrode is made up of porous compressed carbon containing a small amount of catalyst to increase the rate of ORR. The most popular catalyst for the ORR process is platinum; however, the high cost prevents the use of platinum in fuel cells on a commercial scale. Pristine graphene is not effective towards ORR because it is inefficient to enable electron transfer. Because of the charge-polarization of the binding between the dopant and carbon, heteroatom dopants speed up the adsorption of oxygen on the cathode and the breaking of the oxygen–oxygen bond (
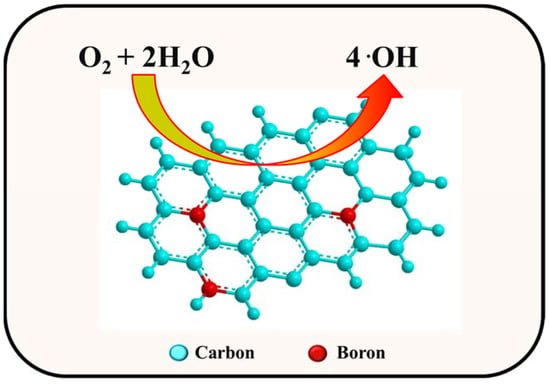
Figure 1).
Figure 1. Illustration of electron transfer for ORR on the P-GNS surface.
The poisons such as carbon monoxide (CO) and methanol (CH
3OH) in electrolyte inhibits the ORR at the platinum surface. This can be avoided by metal-free electrocatalyst such as P-GNS. Phosphorous atoms increase the absorption and cleavage of the oxygen– oxygen bond due to the charge polarization of the heteroatom and carbon bond. Qiao and coworkers reported that dual-doped graphene (PN-GNS) synthesised by pyrolysis of GO (as the carbon source) and diammonium hydrogen phosphate (as the nitrogen and phosphorous source) acted as a better electrocatalyst as compared to single-doped and undoped graphene [
112]. The synergistic effect, fast electron transfer by highly conducting graphene, and the 3D porous structure provided porous channels that made the inner active sites accessible to the oxygen molecules and the electrolyte. Similar results were observed by Jo’s group during the use of BP-GNS as an electrocatalyst in the fuel cells [
113].
The ORR activity of dual-doped graphene can be further improved by their fabrication with inexpensive perovskite such as (La
0.
8Sr
0.
2MnO
3). The introduction of perovskite to undoped graphene increased the number of electrons (n) moved from anode to cathode electrode 2.7 to 3.6 at −0.5 V, but it did not alter the current density. This problem was overcome by fabricating perovskite with dual-doped graphene. Here the n value increased to 3.8 at −0.5 V. This may be due to acceleration or conductivity effects provided by perovskite. The kinetic analysis showed that the direct four-electron pathway was favoured [
114].
With the introduction of phosphorous into co-doped graphene NS-GNS, a large increase in ORR performance was observed because of the formation of a conductive P–N bond. The ternary-doped graphene (PNS-GNS) showed excellent electrocatalytic activity, which was five to six times higher than P-GNS and two times higher than NS-GNS [
115]. A similar method was used by Zhang et al. to use PNF-GNS as an electrocatalyst for fuel cells. Fluorine, a highly electronegative element, is a promising dopant to produce charge polarization that can significantly enhance the electrocatalytic activity for graphitic carbon atoms [
116]. The heteroatom carbon species synergistically affects the performance of dual- and ternary-doped graphene.
Nitrogen-, iron-, sulphur-, and phosphorous-doped graphene was synthesised using the direct pyrolysis of
Shewanella oneidensis bacteria, displaying a comparable catalytic current density to Pt/C [
117]. The presence of metals in an electrocatalyst has been associated with several difficulties, viz. low selectivity, poor stability, a negative environmental impact, and higher cost as compared to metal-free catalysts [
118]. The enhanced ORR activity possessed by the quaternary-doped graphene (BPNS -GNS) vs. the mono-doped graphene was due to two reasons: firstly, the simultaneous insertion of various heteroatoms into the lattice resulted in a synergistic effect; secondly, the ORR activity was strongly influenced by the formation of bonds between different heteroatoms. The P–N moieties formation increased the number of active locations for the adsorption of oxygen gas molecules as compared to the B–N species [
117]. The BPNS-GNS showed outstanding current density values, number of electrons transferred during ORR, and durability as compared to dual- and ternary-doped graphene.
1.2. Lithium-Metal Batteries
Lithium-ion batteries are important energy-producing and storage devices. These are rechargeable batteries, which contain lithium solution as an electrolyte. The importance of lithium-ion batteries in energy-related devices nowadays can be understood from the Nobel Prize given in 2019 to three scientists, Stanley Whittingham, John Goodenough, and Akira Yoshino, for their innovation in the synthesis of lithium-ion batteries. The use of these lightweight, rechargeable, and powerful lithium-ion batteries from electronic gadgets to electric vehicles have revolutionized our lives. The negative electrode of a conventional lithium-ion cell is composed of carbon-based materials, while the positive electrode is a metal oxide. During charging, ions move from anode to cathode and the reverse happens during discharging. Due to its low affinity for lithium atoms and the tendency of deposited lithium atoms to cluster on the graphene surface, pure graphene is not a good choice for lithium storage [
106]. Moreover, the penetration of lithium ions in GNS requires high energy, which can be provided by employing modified graphene and carbon nanomaterials as electrodes. Lithium ions can penetrate graphene materials and lithium clustering is prevented by the presence of defects. Since the lithium atom donates electrons, graphene can be doped with an electron-poor element like boron to boost its storage capacity. However, due to the increased binding energy between lithium and B-GNS, it restricts delithiation (lithium diffusion). In summary, these materials boost battery capacity; however, delithiation is ineffective. Although the storage capacity of graphene is reduced as a result of doping it with electron-rich atoms like nitrogen, phosphorus, and metal oxides, the delithiation process is more effective. This can be caused by reduced electrostatic repulsion between lithium and dopants/ metal oxides and their binding energy. Therefore, the charge/discharge performance is increased by these materials (
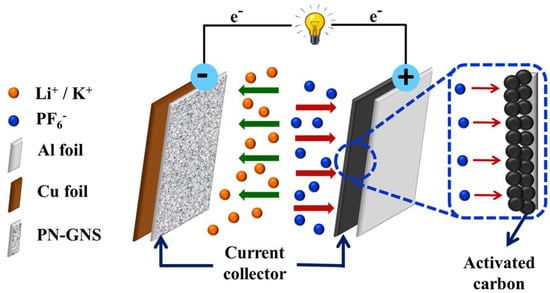
Figure 2).
Figure 2. Schematic diagram of lithium-ion batteries.
Zhang’s group used P-GNS in lithium-ion batteries as an anode material for the first time. They also compared the results with undoped graphene and found that P-GNS acted as a superb electrode because of various topological defects introduced on its surface during the doping process. This led to a randomized carbon lattice that improved the lithium insertion characteristics. P-GNS has a considerably larger reversible discharge and charge capacity than undoped graphene (approximately 280 mAh g
−1), with nearly no loss across 80 cycles, indicating that P-GNS-based electrodes possess a good longevity [
119]. Luan et al. [
120] showed that PN-GNS has a high reversible charge and discharge capacity along with an excellent cycling rate after 600 cycles. The specific capacity of PN-GNS was 889 mAh g
−1 at a current density of 1000 mA g
−1. Due to low abundance and uneven global distribution of lithium ions, the increasing demand of Li-ion batteries can hardly be meet.
1.3. Alkaline Ion Batteries
P-GNS is also a viable option for anodes in sodium-ion batteries [
121]. Na-ion batteries have similar characteristics as Li-ion batteries. Due to its distinct ultrathin and wrinkled shape, P-GNS as an anode material demonstrated superior cycle stability and excellent rate performance. At a current density of 50 mA g
−1, the specific capacity of P-GNS was calculated to be 284.8 mAh g
−1. Due to synergistic effects such as increased interlayer distance, improved electrical conductivity, heteroatomic defects, and good electrode/electrolyte wettability, P-GNS maintained its specific capacity after 600 cycles. A high specific capacity and extremely high cycling stability are both characteristics of graphene with phosphorus and oxygen dual-doping (PO-GNS) (474 mA h g
−1 after 50 cycles at 50 mA g
−1 and 160 mA h g
−1 after 600 cycles at 2000 mA g
−1). This might occur as a result of the dopants’ high inter-layer spacing, which aided the insertion and extraction of potassium ions [
122].
1.4. Supercapacitors
Supercapacitors have high capacitance values as compared to conventional capacitors because they link the gap between rechargeable batteries and electrolytic capacitors. They are also known as gold caps, ultracapacitors, or super caps. Supercapacitors come in a variety of forms, including hybrid capacitors, electrochemical pseudocapacitors, and electrostatic double-layer capacitors. The first type of supercapacitors use carbon materials for electrode preparation, and charge separation is achieved using the Helmholtz double layer at the junction of the electrolyte and the surface of a conductive electrode. In electrochemical pseudocapacitors, conducting polymers and metal oxide electrodes are used and charge separation is achieved by Faradaic electron charge transfer with electrosorption, intercalation, and redox reactions. In the third type, hybrid capacitors are a combination of both electrodes; one that stores the charge faradaically and the other stores the charge electrostatically.
Due to graphene’s enormous surface area and large electric double-layer capacitance, it has recently been employed as an electrode in supercapacitors [
123]. However, pristine graphene is chemically inert, which encounters some challenges as it does not give electrochemical capacitance, i.e., pseudocapacitance [
106]. For usage in supercapacitors, doping can introduce some significant amounts of deviations in the graphene lattice. Heteroatom-doped graphene was more advantageous when used in supercapacitors as it enhanced conductivity, improved stability, and had good reactivity compared to pristine graphene.
Graphene doped with phosphorous exhibited improved capacitive performance in aqueous electrolyte due to higher pseudocapacitance and a strong conducting network in the graphene structure. A supercapacitor was created by Karthika et al. [
124], utilising P-GNS, and it displayed a high power density of 9 kW kg
−1, a high specific capacitance of 367 Fg
−1, and a high energy density of 59 Wh kg
−1. After many hundreds of cycles, there was no discernible loss in any particular capacitance. Thirumal et al. [
82] also synthesised P-GNS using the electrochemical method, where the specific capacitance was 290 Fg
−1 at a current density of 0.5 Ag
−1. Thus, the different approaches affect the capacitance values because different defects are created by the different methods. DFT relates the enhanced specific capacitance of P-GNS with structural changes in the lattice. DFT calculations were used by Song et al. [
79] to identify two distinct mechanisms: first, the rise in interlayer separation following doping; and second, the high polarity of the P–O groups. Electrolyte filled the gap between the graphene sheets as their distance from one another grew, forming additional double layers that may store charge.
Polyaniline–graphene-based electrodes exhibited high specific capacitance of 480 Fg
−1 at a current density of 1 Ag
−1 [
125]. This value can be further enhanced by introducing heteroatoms. Nanocomposite of polyaniline with P-GNS showed high specific capacitance (603 Fg
−1 at 1 Ag
−1), which was six times higher than bare polyaniline. Moreover, this value further increased with an increase in current density from 1 to 15 Ag
−1 [
126].
Because of the synergistic effects of the heteroatom dopants, co-doping of heteroatoms has been seen as a potential way to improve the energy storage performance of graphene-based materials. Excellent specific capacitance of 183 Fg
−1 at 0.05 Ag
−1 current density was present in PN-GNS. P-GNS is a superior supercapacitor electrode than a broad potential window of 1.6 V in an aqueous electrolyte because it contains functional groups that include phosphorus [
127].
2. Applications of B-GNS
The applications of B-GNS are discussed in different subsections, viz. solar cells, sensors, supercapacitors, lithium-ion batteries, fuel cells, and photocatalysts. The properties of the materials are briefly discussed in next subsections.
2.1. Fuel Cells
The substituted boron atoms in the graphene lattice acted as active sites for adsorption of oxygen and speeding up oxygen–oxygen bond breaking. Molina-Garcia et al. [
114] observed that binary-doped graphene improved conductivity, making it a suitable catalyst for ORR activity. This might be the result of flaws that developed as a result of the doped graphene, which increased the electrical conductivity of the perovskite materials in the end. The kinetic results depicted that the four-electron pathway was followed. As an alternative to Pt-based materials with high costs, multiple doping of graphene can be used to create an electrocatalyst that is affordable, dependable, and metal free. Co-doping graphene with various atoms may help it perform even better electrocatalytically in the ORR process.
Wu et al. [
128] carried out a structural modification of graphene with boron and nitrogen dopants using the hydrothermal self-assembly method. The experimental results revealed that edge-rich BN-GNS without an inert covalent B–N bond showed more superior ORR electrocatalytic performance as compared to BN-GNS with fewer edges and an inert B–N covalent bond [
128]. Qin et al. [
83] prepared BN-GNS to study the adsorption and reduction of oxygen on a cathode using DFT studies. Their calculations indicated that the BC
3 and graphitic N were the main active sites among various boron doping or/and nitrogen configurations. Mazanek et al. [
1] also synthesised BN-GNS using thermal exfoliation of GO and studied its electrocatalytic performance for ORR process. They determined the atomic percentage of boron and nitrogen using ICAP-AES analysis. The boron content (4.33 to 6.53%) was higher than the nitrogen content (0.11 to 0.53%). They found that ORR performance was related to intrinsic doping as well as metallic impurities introduced during the synthesis of GO, such as manganese, chlorate, permanganate, etc. In order to use BN-GNS as a metal-free electrocatalyst in fuel cells, Il and colleagues synthesised BN-GNS. The electron transfer number (n) in the 0.225–0.465 V potential range was 3.53–3.84, indicating that the BN–GNS preferred the four-electron pathway for oxygen reduction. These findings showed that doped graphene had the potential to take the place of pricey precious metal catalysts [
129].
With a high current density (55 A cm
−1) and a low onset potential (−0.10 V), BNP-GNS demonstrated excellent electrocatalytic activity [
130]. Due to the incorporation of various dopants with various electronegativities, quaternary-doped graphene (BPNS-GNS) demonstrated superior ORR catalytic activity in comparison to single- and dual-doped graphenes. They were able to change the oxygen molecule’s binding energy, which made oxygen dissociation easier [
117].
2.2. Solar Cells
The solar cell is an electrical device that changes sunlight or artificial light into electrical energy through the photovoltaic effect and thus it is also known as the photovoltaic cell. The absorption of light energy, which produces excitons or electron–hole pairs, is one of the three fundamental characteristics needed for a solar cell to function. The separation of charge carriers of different types is the second step, and the independent extraction of those carriers to an external circuit is the third step. Dye-sensitized, organic, quantum dot, perovskite, and perovskite solar cells are examples of common solar cells. The four layers of a solar cell are the interface layer (Au, indium tin oxide), hole transport layer (2,2′,7,7′-tetrakis (N,N-di-4-methoxy-phenylamino)-9,9′,-spiro bi fluorene), electron transport layer (TiO
2), and transparent conducting layer (fluorine tin oxide) [
91]. The power conversion efficiency (PCE), which is determined by the percentage of incident power converted into electricity, is used to calculate the total efficiency of solar cells. In solar cells with PCE of 7.09–8.96%, p-type material like B-rGO serves as a hole transport layer [
91]. A B-GNS/SiO
2-based solar cell with an open current voltage of 0.53 V, and a short current density of 18.8 mA cm
−2 was developed by Li et al. [
101] under one solar irradiation. A nitric acid treatment of B-GNS considerably improved the performance of the solar cell. Nitric ions added more P-doping, improving the electrical conductivity, and decreasing the charge transfer resistance [
101,
131]. These studies imply that doped graphene can function as an efficient hole transport material in the active layer of solar cells.
2.3. Lithium-Metal Batteries
Due to their low memory loss, high energy density, and low self-discharge rate, rechargeable lithium-ion batteries are extensively employed in portable electronic devices [
132]. Using a hydrogen-assisted reduction process, Sahoo et al. created SnO
2-adorned B-GNS for use as a lithium-ion battery anode material. In comparison to bare SnO
2 and B-GNS, they discovered that the synthesised hybrid had the largest reversible capacity of 744 mAh g
−1 at a current density of 100 mAh g
−1. An improved lithium interaction resulted from the SnO
2, a spacer within the GNS, increasing the likelihood of lithium intercalation and alloying against re-stacking of the 2D planes. Due to the wrinkled graphene network and homogenous dispersion of tiny SnO
2 nanoparticles (NPs), a rise in reversible capacity was seen with increasing boron content in B-GNS [
23].
Silver (Ag) NPs embedded on B-rGO served as the anode material in a nanocomposite that was synthesised by Bindumandhavan et al. [
64]. Due to the interaction between B-GNS and Ag NPs, this type of anode material demonstrated a substantial reversible capacity (1484 mAh g
−1 at 50 mA g
−1). This anode showed increased cycle stability and reversible capacity of 540 mAh g
−1 at 100 mAh g
−1 after 100 consecutive cycles. The presence of defect sites and heteroatoms in the presence of boron and Ag NPs was attributed to these outstanding electrochemical performances. Tian et al. [
133] have also explored the possibilities of boron- and sulphur-doped reduced graphene oxide (BS-rGO) as a cathode in lithium-ion batteries. According to their findings, a hydrothermally produced BS-rGO cathode outperformed S-rGO in terms of reversible discharge capacity, achieving 521 mAh g
−1 at 0.1 C after 100 cycles. The excellent high rate of performance was attributed to the B-strong rGO’s electrical conductivity. The composite cathode’s electric conductivity was enhanced and the shuttle effect was delayed by boron doping on rGO. Due to the co-dopants’ synergistic effects, BN-GNS produced by the hydrothermal process performed better than single-doped graphene. For the objective of preserving active material during the cycle and enhancing the carbon’s wettability in the organic electrolyte, borax and nitrogen dopants had significant binding opportunities with polysulfide species [
134]. The next-generation energy storage technology for lithium-sulfur batteries is thought to be promising [
135].
2.4. Alkaline Ion Batteries
Potassium-ion batteries are gaining popularity as lithium-ion battery substitutes due to the abundant availability of potassium in the Earth’s crust. B-GNS has the potential to be used as an anode material for potassium-ion batteries due to its high specific capacity of 546 mAh g
−1 and low migration barrier of 0.07 eV. This may be due to the substrate being doped with boron, which causes it to become electron-deficient and causes a sizable charge transfer from potassium to the substrate. Due to the inhibition of dendritic development and prevention of potassium atom clustering, there is good cycling stability [
136,
137].
2.5. Supercapacitors
Supercapacitors based on doped graphene electrodes showed higher interfacial capacitance as compared to undoped ones. Some researchers reported that the presence of dopant on the graphitic lattice might lead to electrochemical reduction–oxidation reactions on the surface, which would ultimately enhance the capacitance of the material. The specific capacitance of boron-doped reduced GO was 448 Fg
−1, which was three times higher than reduced GO (135 Fg
−1). A supercapacitor’s specific capacitance typically depends on the electrode’s BET surface area and electrical conductivity, both of which decrease as the annealing temperature rises. On the other hand, boron-doped GO lowered the electrical conductivity and BET surface area increased as the annealing temperature rose from 300 to 1000 °C. At a higher temperature (1000 °C), the doping of boron heteroatoms may create defect-like small pores in the graphene nanosheet’s plane that prevents the growth of graphitic structure [
94].
Thirumal et al. performed galvanometric charge–discharge and cyclic voltammetry (CV) measurements on thermally reduced graphene and B-GNS in 0.5 M H
2SO
4 at various current densities (1 Ag
−1 to 4 Ag
−1). The curves of charge–discharge were triangular in shape, and CV curves were rectangular in shape, which suggested that both electrodes showed double-layer capacitance behaviour. The highest specific capacitance for thermally reduced graphene and B-GNS was 52 and 113 Fg
−1, respectively, which showed that B-GNS can act as a better electrode as compared to undoped GNS for supercapacitor applications [
95]. However, Mombeshora et al. reported contradicting results [
138]. This research group prepared B-GNS using the thermal reduction method in the presence of sodium borohydride [
138]. During the reduction process, defects were created that acted as charge-trapping sites.
Numerous publications also examined the effects of co-doping graphene with two or more heteroatoms. Chen et al. produced BN-GNS with atoms ranging from 0.6% to 2.1% (B) and 1.74 to 2.56% (N). In order to get a satisfactory performance in electrochemical energy storage, dopants were simultaneously introduced, creating a synergistic effect between boron and nitrogen [
97]. The findings indicated that while boron doping enhances the performance of supercapacitors at high current densities, nitrogen doping can guarantee high capacitance.
This entry is adapted from the peer-reviewed paper 10.3390/ma16031155