One of the most advanced, promising and commercially feasible research issues in the field of hydrogel dressings is to obtain functions to achieve improved therapeutic effects and even intelligent wound repair. In addition to the advantages of ordinary hydrogel dressings, functional hydrogel dressings can also adjust their chemical/physical properties to meet different wound types, respond accordingly, actively create a healing environment conducive to wound repair, and control drug release to provide lasting benefits.
- hydrogel dressing
- functionality
- wound repair
1. Dynamic cross-linked hydrogel dressing
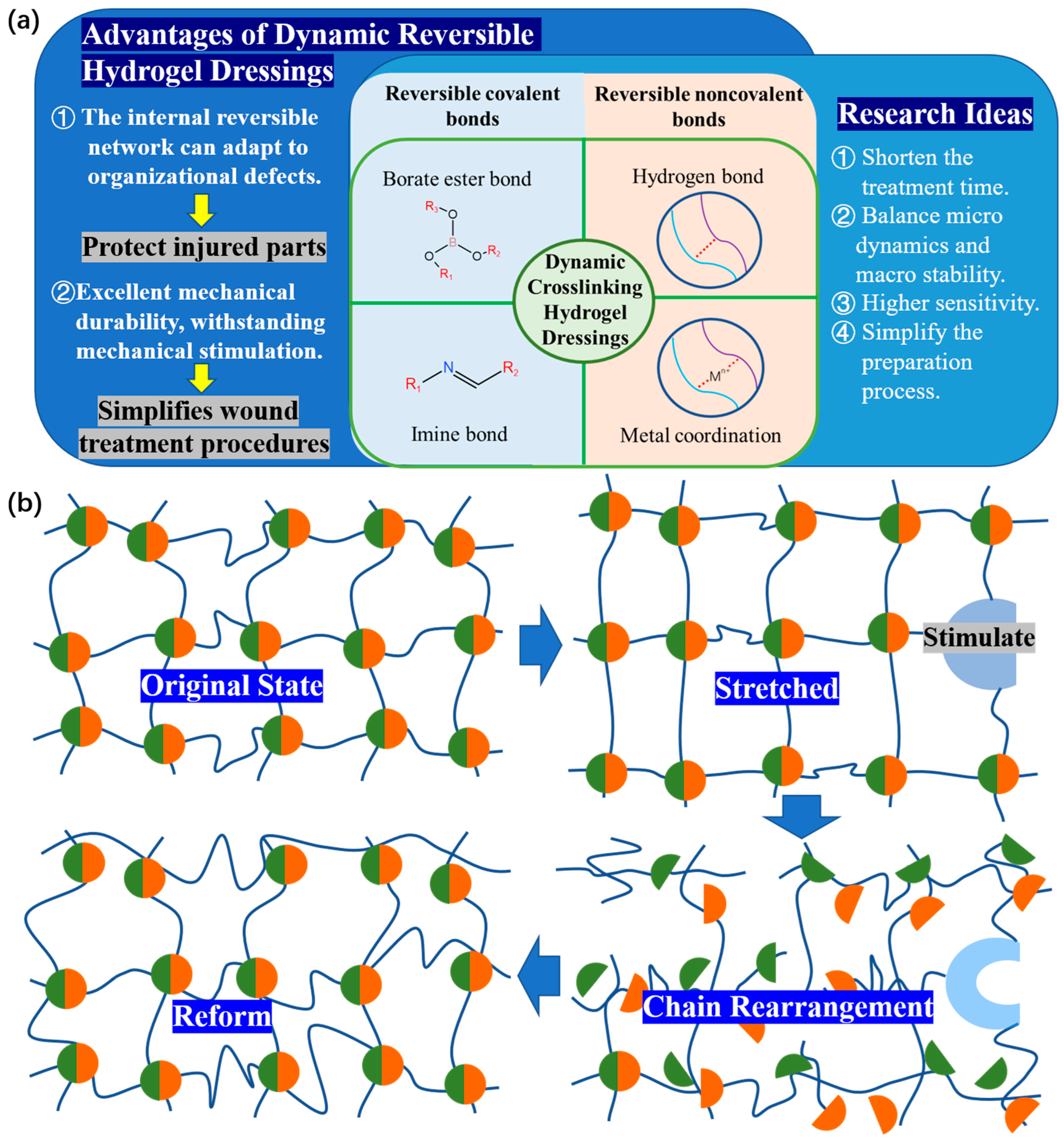
When discussing the general expectations and design principles of the performance of dynamic hydrogel dressings, the balance between the macro stability and micro level dynamics of hydrogel dressings and the application requirements of dressings should be considered. In practice, the most common reversible covalent bond is the imine bond. The borate ester bond, a special dynamic covalent bond, can exchange bonds within a few seconds at room temperature [9]. Metal coordination bond and hydrogen bond are two typical examples of reversible noncovalent bond. This will serve as the starting point for this section, introducing the success of current research in this field.
2. Reversible covalent bond
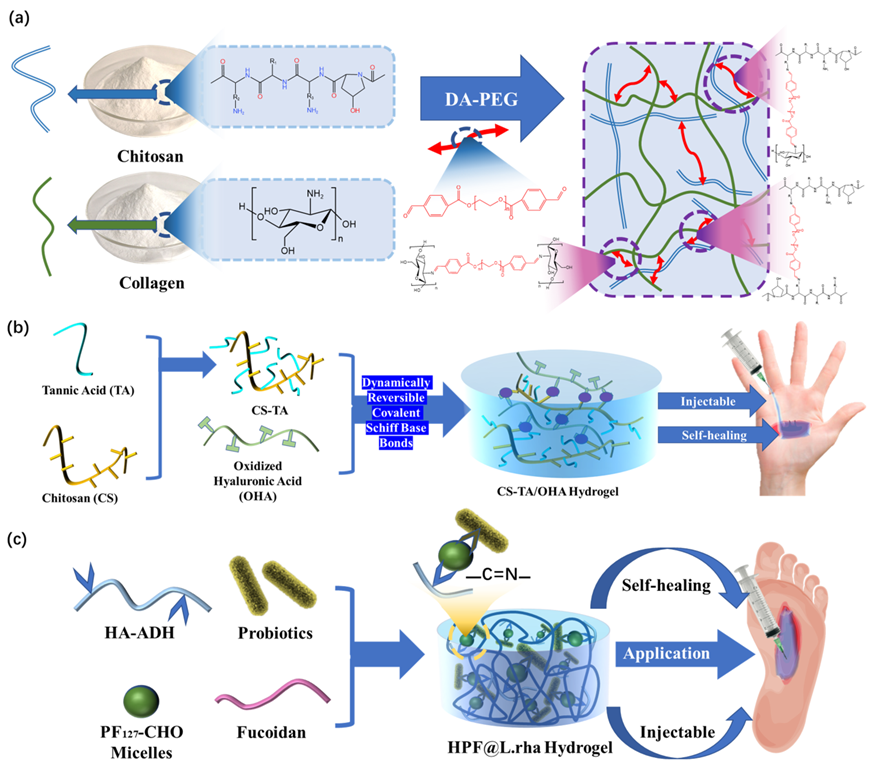
2.1. Imine bond
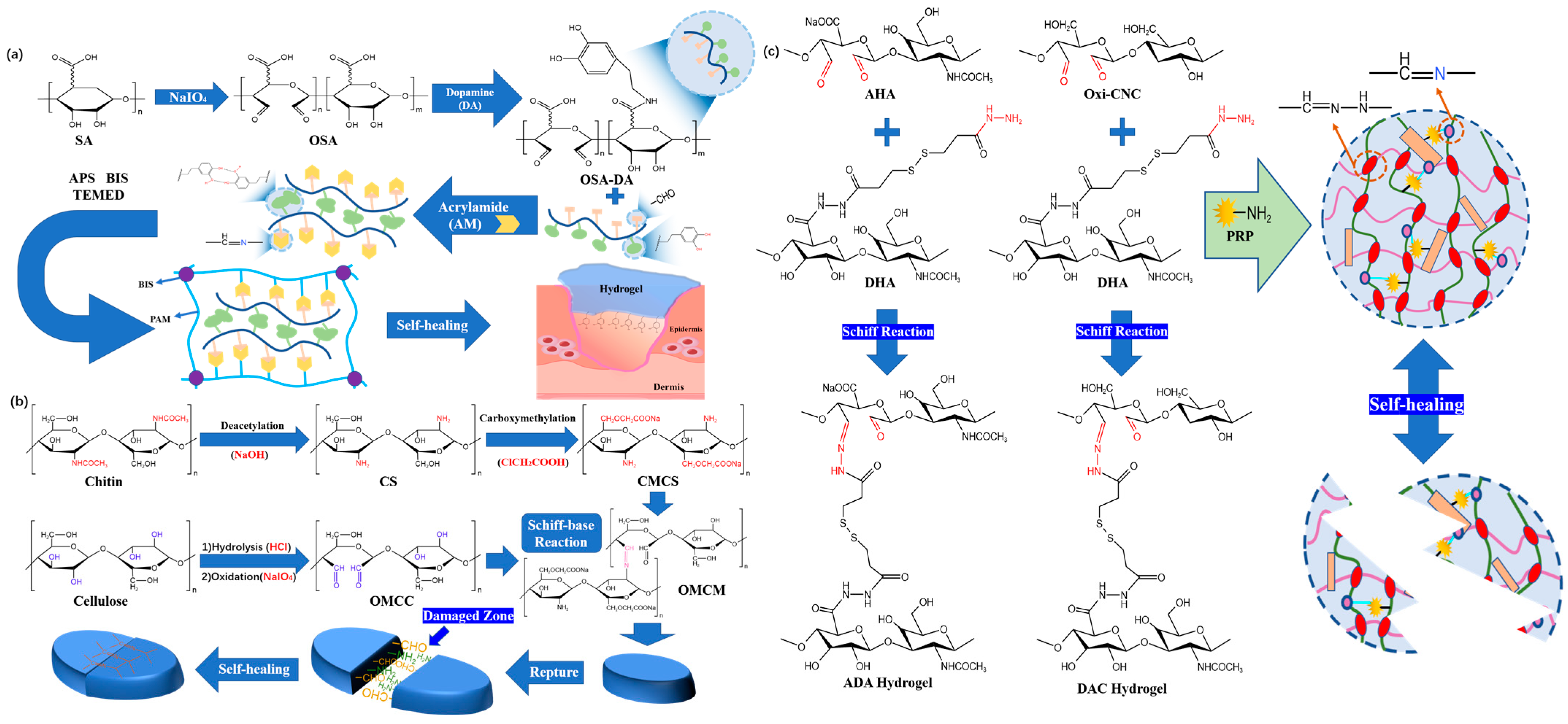
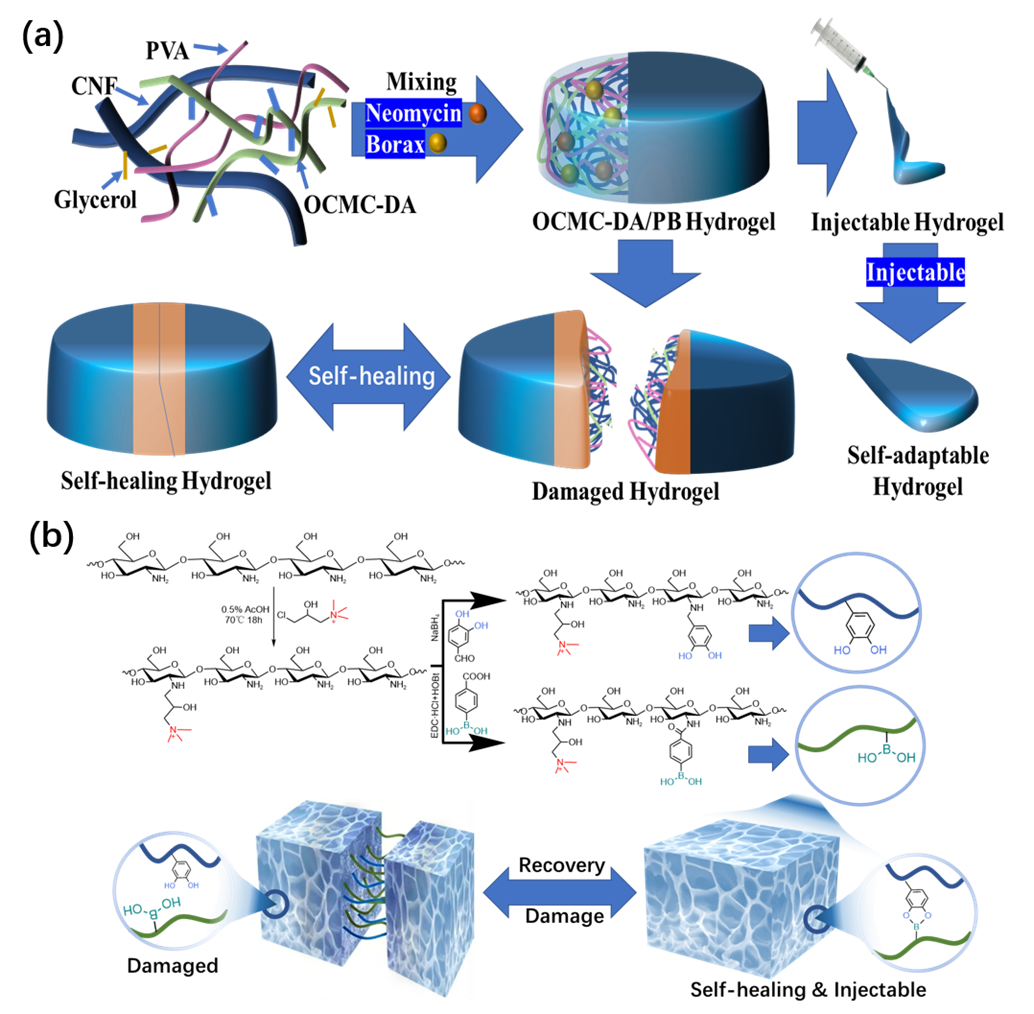
2.2. Boric Acid Ester Bonds
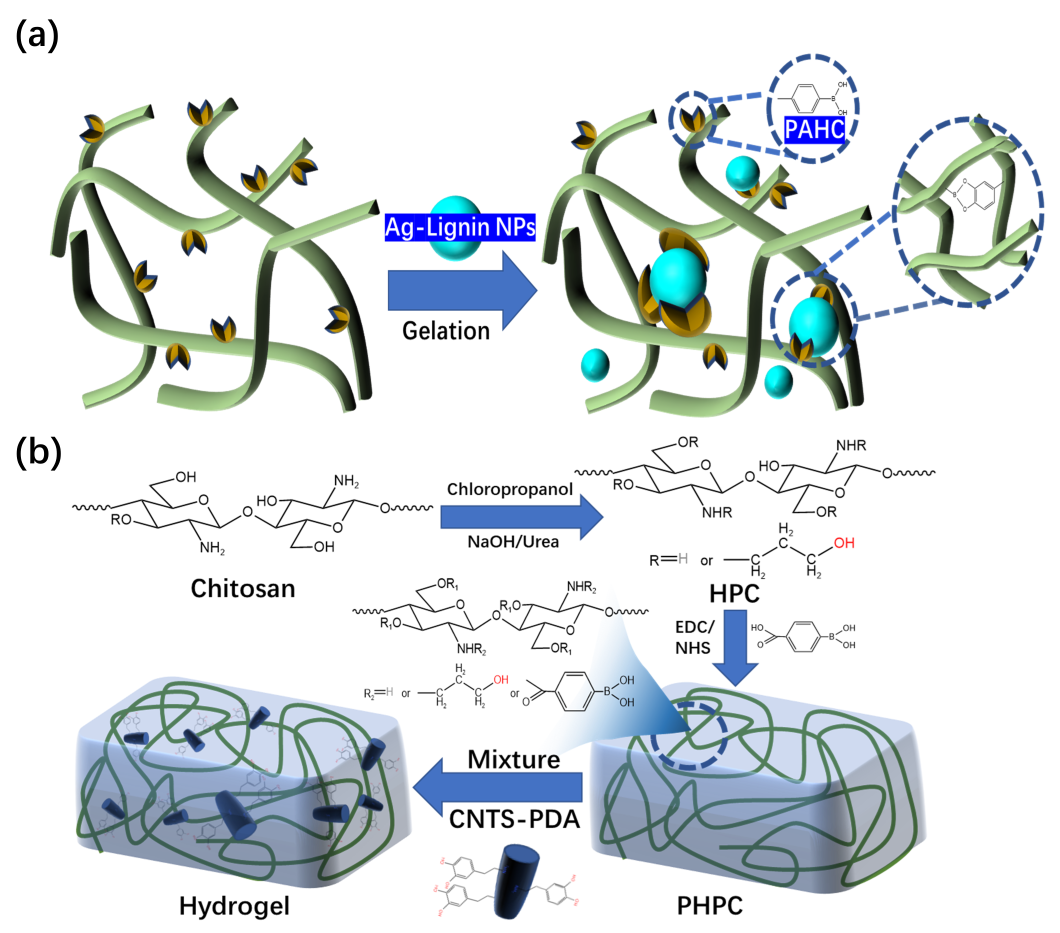
3. Reversible Noncovalent Bonds
3.1. Metal Coordination Bond
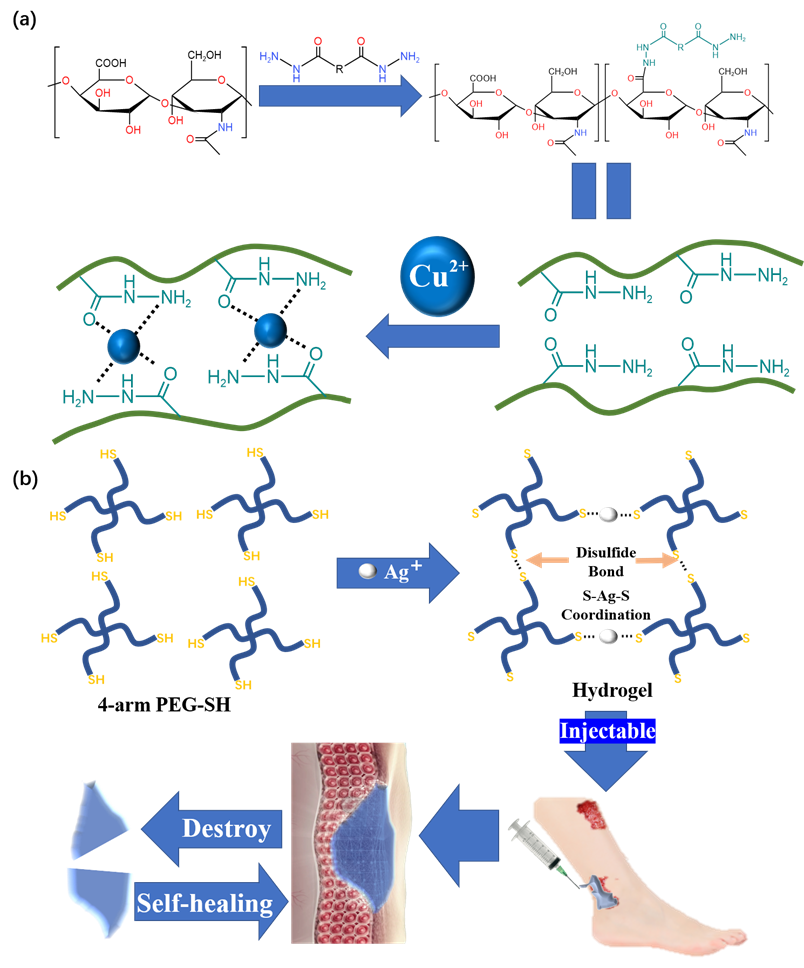
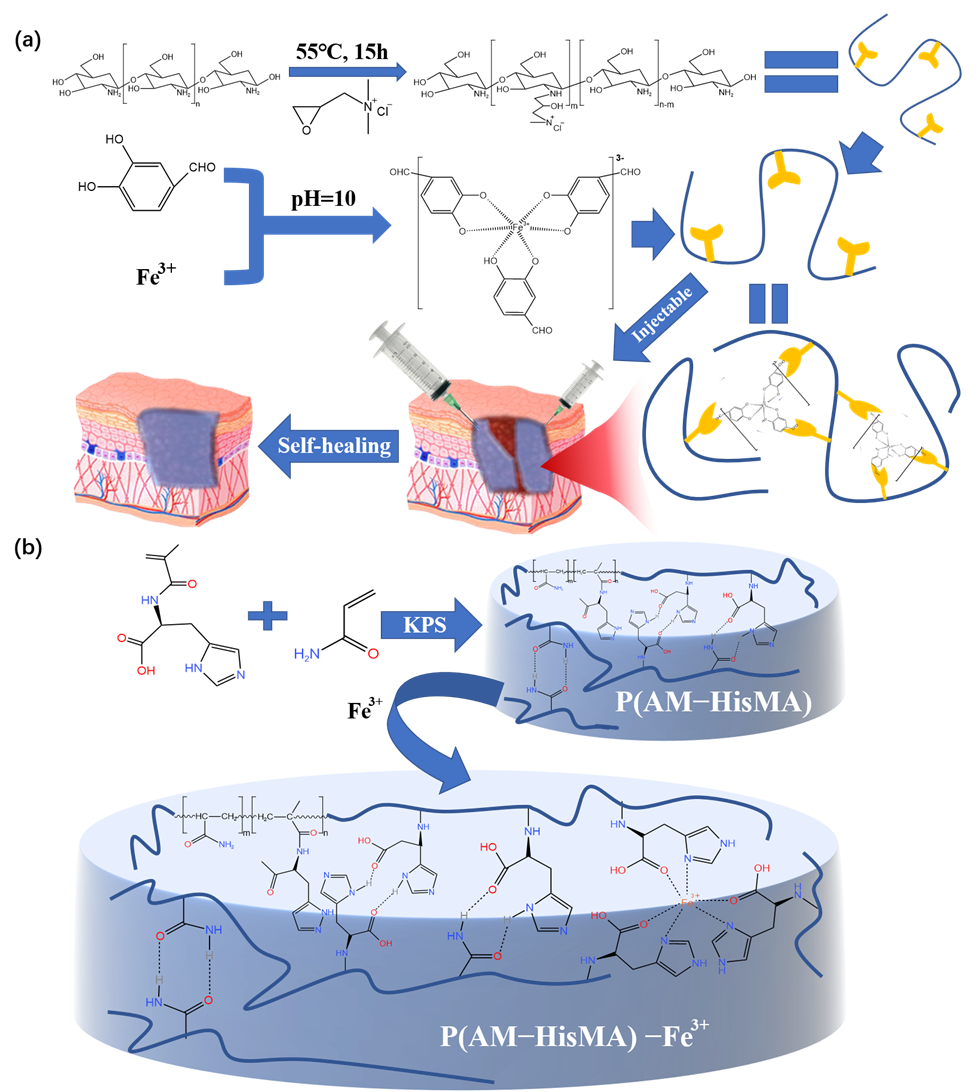
3.2. Hydrogen Bond
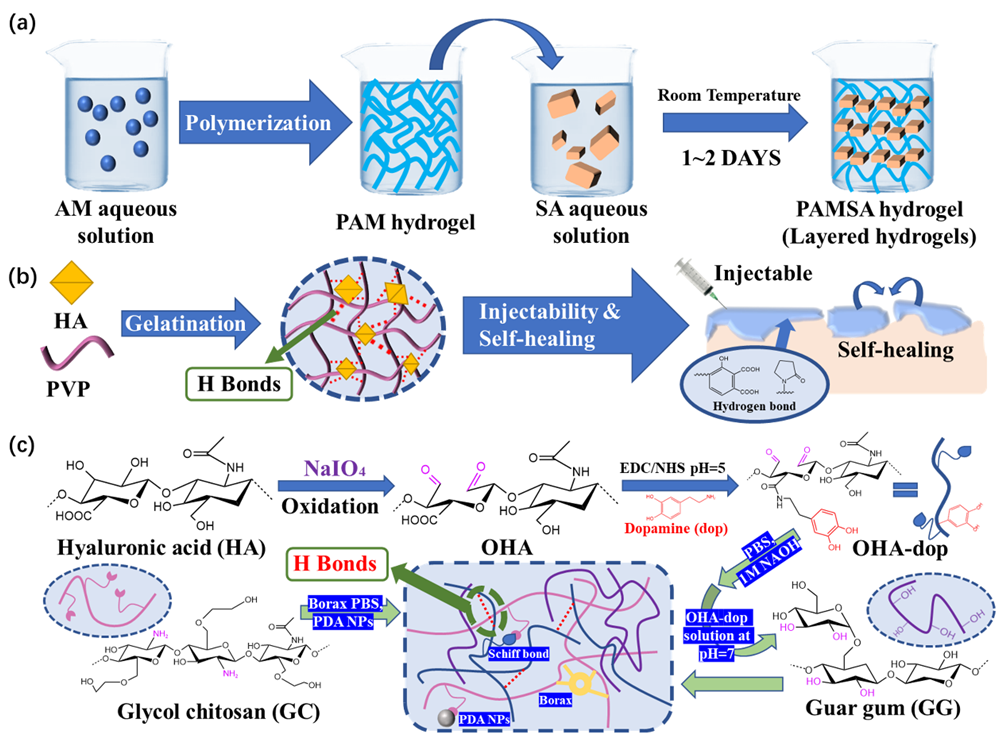
This entry is adapted from the peer-reviewed paper 10.3390/polym15092000
References
- Ding, X.Y.; Yu, Y.R.; Zu, Y. Self-healing hydrogels based on the Knoevenagel condensation reaction for wound healing. Biomed. Technol. 2023, 2, 70–76.
- Deng, Z.X.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B.L. Multifunctional stimuli-responsive hydrogels with self-Healing, high conductivity, and rapid recovery through host–guest interactions. Chem. Mater. 2018, 30, 1729–1742.
- Han, W.; Chen, C.; Yang, K.; Wang, H.B.; Xia, H.G.; Zhao, Y.; Teng, Y.; Feng, G.C.; Chen, Y.M. Hyaluronic acid and chitosan-based injectable and self-healing hydrogel with inherent antibacterial and antioxidant bioactivities. Int. J. Biol. Macromol. 2023, 227, 373–383.
- Gao, G.R.; Yang, F.J.; Zhou, F.H.; He, J.; Lu, W.I.; Xiao, P.; Yan, H.Z.; Pan, C.F.; Chen, T.; Wang, Z.L. Bioinspired self-healing human-machine interactive touch pad with pressure-sensitive adhesiveness on targeted substrates. Adv. Mater. 2020, 32, 2004290.
- Wang, Z.Y.; Gu, J.Y.; Zhang, D.F.; Zhang, Y.; Chen, J.H. Structurally dynamic gelatin-based hydrogels with self-healing, shape memory, and cytocompatible properties for 4D printing. Biomacromolecules 2023, 24, 109–117.
- Zhang, Z.; Abidi, N.; Lucia, L.A. Dual crosslinked-network self-healing composite hydrogels exhibit enhanced water adaptivity and reinforcement. Ind. Eng. Chem. Res. 2022, 61, 17876–17884.
- Feng, W.J.; Wang, Z.K. Shear-thinning and self-healing chitosan-graphene oxide hydrogel for hemostasis and wound healing. Carbohydr. Polym. 2022, 294, 119824.
- Zhang, K.Y.; Feng, Q.; Fang, Z.W.; Gu, L.; Bian, L.M. Structurally dynamic hydrogels for biomedical applications: Pursuing a fine balance between macroscopic stability and microscopic dynamics. Chem. Rev. 2021, 121, 11149–11193.
- Cromwell, O.R.; Chung, J.; Guan, Z.B. Malleable and self-Healing covalent polymer networks through tunable dynamic boronic ester bonds. J. Am. Chem. Soc. 2015, 137, 6492–6495.
- Ikura, R.; Park, J.; Osaki, M.; Yamaguchi, H.; Harada, A.; Takashima, Y. Design of self-healing and self-restoring materials utilizing reversible and movable crosslinks. NPG Asia Mater. 2022, 14, 10.
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Untersuchungen ü das Chinolin. Justus Liebigs Ann. Chem. 1864, 131, 118–119.
- Pogostin, B.H.; Saenz, G.; Cole, C.C.; Euliano, E.M.; Hartgerink, J.D.; McHugh, K.J. Dynamic imine bonding facilitates mannan release from a nanofibrous peptide hydrogel. Bioconjug. Chem. 2023, 34, 193–203.
- Min, J.B.; Zhou, Z.X.; Wang, H.N.; Chen, Q.H.; Hong, M.C.; Fu, H.Q. Room temperature self-healing and recyclable conductive composites for flexible electronic devices based on imine reversible covalent bond. J. Alloys Compd. 2022, 894, 162433.
- Chen, T.; Chen, Y.J.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H.Z. Ultratough self-healing and tissue-adhesive hydrogel for wound dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531.
- Yin, H.S.; Song, P.Q.; Chen, X.Y.; Huang, Q.Y.; Huang, H.H. A self-healing hydrogel based on oxidized microcrystalline cellulose and carboxymethyl chitosan as wound dressing material. Int. J. Biol. Macromol. 2022, 221, 1606–1617.
- Nie, L.; Wei, Q.Q.; Sun, M.; Ding, P.; Wang, L.; Sun, Y.F.; Ding, X.Y.; Okoro, O.V.; Jiang, G.H.; Shavandi, A. Injectable, self-healing, transparent, and antibacterial hydrogels based on chitosan and dextran for wound dressings. Int. J. Biol. Macromol. 2023, 233, 123494.
- Li, S.Z.; Dong, Q.; Peng, X.T.; Chen, Y.; Yang, H.J.; Xu, W.L.; Zhao, Y.T.; Xiao, P.; Zhou, Y.S. Self-healing hyaluronic acid nanocomposite hydrogels with platelet-rich plasma impregnated for skin regeneration. ACS Nano 2022, 16, 11346–11359.
- Ding, C.C.; Tian, M.D.; Feng, R.; Dang, Y.; Zhang, M. Novel self-healing hydrogel with injectable, pH-responsive, strain-sensitive, promoting wound-healing, and hemostatic properties based on collagen and chitosan. ACS Biomater. Sci. Eng. 2020, 6, 3855–3867.
- Zhang, X.; Tan, B.W.; Wu, Y.T.; Zhang, M.; Xie, X.; Liao, J.F. An injectable, self-healing carboxymethylated chitosan hydrogel with mild photothermal stimulation for wound healing. Carbohydr. Polym. 2022, 293, 119722.
- Mei, L.; Zhang, D.J.; Shao, H.R.; Hao, Y.P.; Zhang, T.; Zheng, W.P.; Ji, Y.J.; Ling, P.X.; Lu, Y.; Zhou, Q.H. Injectable and self-healing probiotics-loaded hydrogel for promoting superbacteria-infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 20538–20550.
- Liu, S.X.; Jiang, N.; Chi, Y.Q.; Peng, Q.; Dai, G.R.; Qian, L.; Xu, K.M.; Zhong, W.Y.; Yue, W.Q. Injectable and self-Healing hydrogel based on chitosan-tannic acid and oxidized hyaluronic acid for wound healing. ACS Biomater. Sci. Eng. 2022, 8, 3754–3764.
- Chen, M.T.; Wu, Y.; Chen, B.H.; Tucker, A.M.; Jagota, A.; Yang, S. Fast, strong, and reversible adhesives with dynamic covalent bonds for potential use in wound dressing. Proc. Natl. Acad. Sci. USA 2022, 119, e2203074119.
- Ivanov, A.E.; Larsson, H.; Galaev, I.Y.; Mattiasson, B. Synthesis of boronate-containing copolymers of N,N-dimethylacrylamide, their interaction with poly(vinyl alcohol) and rheological behaviour of the gels. Polymer 2004, 45, 2495–2505.
- Chen, Y.M.; Qian, W.Q.; Chen, R.; Zhang, H.J.; Li, X.J.; Shi, D.J.; Dong, W.F.; Chen, M.Q.; Zhao, Y. One-pot preparation of autonomously self-healable elastomeric hydrogel from boricacid and random copolymer bearing hydroxyl groups. ACS Macro Lett. 2017, 6, 1129–1133.
- Zhong, Y.J.; Seidi, F.; Li, C.C.; Wan, Z.M.; Jin, Y.C.; Song, J.L.; Xiao, H.N. Antimicrobial/biocompatible hydrogels dual-reinforced by cellulose as ultrastretchable and rapid self-Healing wound dressing. Biomacromolecules 2021, 22, 1654–1663.
- Zhong, Y.J.; Seidi, F.; Wang, Y.L.; Zheng, L.; Jin, Y.C.; Xiao, H.N. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103.
- Deng, P.P.; Chen, F.X.; Zhang, H.D.; Chen, Y.; Zhou, J.P. Conductive, Self-Healing, adhesive, and antibacterial hydrogels based on lignin/cellulose for rapid MRSA-infected wound repairing. ACS Appl. Mater. Interfaces 2021, 13, 52333–52345.
- Deng, P.P.; Liang, X.; Chen, F.X.; Chen, Y.; Zhou, J.P. Novel multifunctional dual-dynamic-bonds crosslinked hydrogels for multi-strategy therapy of MRSA-infected wounds. Appl. Mater. Today 2022, 26, 101362.
- Xue, K.; Liow, S.S.; Karim, A.A.; Li, Z.B.; Loh, X.J. A recent perspective on noncovalently formed polymeric hydrogels. Chem. Rec. 2018, 18, 1517–1529.
- Hou, B.N.; Shen, H.L.; Li, J.; Xie, W.Q.; Li, Z.Z. Self-healing polymer hydrogel based on dynamic chemical bonds. J. Mater. Eng. 2020, 48, 73–82.
- Qian, J.M.; Ji, L.J.; Xu, W.J.; Hou, G.H.; Wang, J.L.; Wang, Y.P.; Wang, T.B. Copper-hydrazide coordinated multifunctional hyaluronan hydrogels for infected wound healing. ACS Appl. Mater. Interfaces 2022, 14, 16018–16031.
- Rather, R.A.; Sarwara, R.K.; Das, N.; Pal, B. Impact of reducing and capping agents on carbohydrates for the growth of Ag and Cu nanostructures and their antibacterial activities. Particuology 2019, 43, 219–226.
- Chen, H.; Cheng, R.Y.; Zhao, X.; Zhang, Y.H.; Tam, A.; Yan, Y.F.; Shen, H.K.; Zhang, Y.S.; Qi, J.; Feng, Y.H.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3.
- Hu, J.L.; Feng, K.K.; Cong, Y.Y.; Li, X.Y.; Jiang, Y.J.; Jiao, X.D.; Li, Y.R.; Zhang, Y.Q.; Dong, X.Y.; Lu, W.F.; et al. Nanosized shikonin-Fe(III) coordination material for synergistic wound treatment: An initial explorative study. ACS Appl. Mater. Interfaces 2022, 14, 56510–56524.
- Liang, Y.Q.; Li, Z.L.; Huang, Y.; Yu, R.; Guo, B.L. Dual-dynamic-bond cross-linked antibacterial adhesive hydrogel sealants with on-demand removability for post-wound-closure and infected wound healing. ACS Nano 2021, 15, 7078–7093.
- Zhang, H.; He, J.D.; Qu, J.Q. Metal-coordinated amino acid hydrogels with ultra-stretchability, adhesion, and self-healing properties for wound healing. Eur. Polym. J. 2022, 179, 111548.
- Devi VK, A.; Shyam, R.; Palaniappan, A.; Jaiswal, A.K.; Oh, T.-H.; Nathanael, A.J. Self-Healing Hydrogels: Preparation, Mechanism and Advancement in Biomedical Applications. Polymers 2021, 13, 3782.
- Zhao, D.W.; Feng, M.; Zhang, L.; He, B.; Chen, X.Y.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580.
- Yu, H.; Xiao, Q.H.; Qi, G.L.; Chen, F.X.; Tu, B.Y.; Zhang, S.; Li, Y.P.; Chen, Y.; Yu, H.; Duan, P. A hydrogen bonds-crosslinked hydrogels with self-healing and adhesive properties for hemostatic. Front. Bioeng. Biotechnol. 2022, 10, 855013.
- Guo, H.L.; Huang, S.; Xu, A.; Xue, W. Injectable adhesive self-healing multiple-dynamic-bond crosslinked hydrogel with photothermal antibacterial activity for infected wound healing. Chem. Mater. 2022, 34, 2655–2671.
