Probiotics have been extensively studied within the medical, pharmaceutical, and food fields, as it has been revealed that these microorganisms can provide health benefits from their consumption. Bacterial probiotics comprise species derived from lactic acid bacteria (LAB) (genus Lactobacillus, Leuconostoc, and Streptococcus), the genus Bifidobacterium, and strains of Bacillus and Escherichia coli, among others. The growth of bacterial probiotics is involved in fermentation, which metabolizes the nutrients of the medium and produces a wide variety of metabolic compounds. Probiotics are defined as microorganisms that, when consumed in adequate doses or concentrations, can benefit the consumer’s health.
- probiotic bacteria
- metabolites
- health effects
1. Introduction
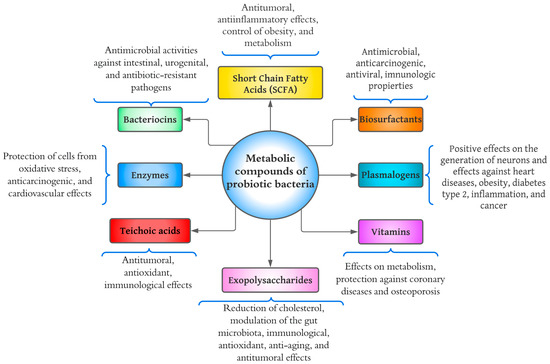
2. Short-Chain Fatty Acids
3. Plasmalogens
4. Enzymes
5. Bacteriocins
-
Class I: Small proteolytic and heat-resistant peptides substantially modified by transcriptionally specific enzymes. Examples: lantibiotics (nisin), sactipeptide, and loop peptides [18].
-
Class II: Divided into four subtypes that are (1) pediocin-like, (2) two peptides, (3) circular, and (4) linear, not pediocin-like. They comprise small peptides resistant to temperature and pH [18].
-
Class III: Large thermolabile peptides (>30 kDa) with complex activity and structure. This group includes helveticin, acidophylline, and lactacins (A and B) [19].
-
Class V: Peptides with circular structures without post-translational modifications, including enterokine AS-48 and gasericin A [19].
6. Exopolysaccharides (EPSs)
7. Teichoic Acids
8. Vitamins
9. Biosurfactants
This entry is adapted from the peer-reviewed paper 10.3390/molecules28031230
References
- Aguilar-Toalá, J. E.; Garcia-Varela, R.; Garcia, H. S.; Mata-Haro, V.; González-Córdova, A. F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. https://doi.org/10.1016/j.tifs.2018.03.009.
- Stiles, M. E. Biopreservation by Lactic Acid Bacteria. Antonie Van Leeuwenhoek 1996, 70 (2–4), 331–345. https://doi.org/10.1007/BF00395940.
- Nataraj, B. H.; Ali, S. A.; Behare, P. V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and Functional Foods. Cell Factories 2020, 19 (1), 168. https://doi.org/10.1186/s12934-020-01426-w.
- Erginkaya, Z.; Konuray-Altun, G. Potential Biotherapeutic Properties of Lactic Acid Bacteria in Foods. Food Biosci. 2022, 46, 101544. https://doi.org/10.1016/j.fbio.2022.101544.
- Hernández-Granados, M. J.; Franco-Robles, E. Postbiotics in Human Health: Possible New Functional Ingredients? Food Res. Int. 2020, 137, 109660. https://doi.org/10.1016/j.foodres.2020.109660.
- Morrison, D. J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7 (3), 189–200. https://doi.org/10.1080/19490976.2015.1134082.
- Gill, P. A.; van Zelm, M. C.; Muir, J. G.; Gibson, P. R. Review Article: Short Chain Fatty Acids as Potential Therapeutic Agents in Human Gastrointestinal and Inflammatory Disorders. Pharmacol. Ther. 2018, 48 (1), 15–34. https://doi.org/10.1111/apt.14689.
- LeBlanc, J. G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L. G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Cell Factories 2017, 16 (1), 79. https://doi.org/10.1186/s12934-017-0691-z.
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; Parmentier, M.; Detheux, M. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. Biol. Chem. 2003, 278 (28), 25481–25489. https://doi.org/10.1074/jbc.M301403200.
- Gill, P. A.; Bogatyrev, A.; Zelm, M. C.; Gibson, P. R.; Muir, J. G. Delivery of Acetate to the Peripheral Blood after Consumption of Foods High in Short‐Chain Fatty Acids. Nutr. Food Res. 2021, 65 (4), 2000953. https://doi.org/10.1002/mnfr.202000953.
- Řezanka, T.; Křesinová, Z.; Kolouchová, I.; Sigler, K. Lipidomic Analysis of Bacterial Plasmalogens. Folia Microbiol. (Praha) 2012, 57 (5), 463–472. https://doi.org/10.1007/s12223-012-0178-6.
- Wallner, S.; Schmitz, G. Plasmalogens the Neglected Regulatory and Scavenging Lipid Species. Phys. Lipids 2011, 164 (6), 573–589. https://doi.org/10.1016/j.chemphyslip.2011.06.008.
- Oberg, T. S.; Ward, R. E.; Steele, J. L.; Broadbent, J. R. Identification of Plasmalogens in the Cytoplasmic Membrane of Bifidobacterium Animalis Subsp. Lactis. Environ. Microbiol. 2012, 78 (3), 880–884. https://doi.org/10.1128/AEM.06968-11.
- Han, X.; Holtzman, D. M.; McKeel, D. W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry: Plasmalogen Deficiency in Alzheimer’s Disease. Neurochem. 2001, 77 (4), 1168–1180. https://doi.org/10.1046/j.1471-4159.2001.00332.x.
- Hossain, Md. S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral Ingestion of Plasmalogens Can Attenuate the LPS-Induced Memory Loss and Microglial Activation. Biophys. Res. Commun. 2018, 496 (4), 1033–1039. https://doi.org/10.1016/j.bbrc.2018.01.078.
- Contesini, F. J.; Melo, R. R. de; Sato, H. H. An Overview of Bacillus Proteases: From Production to Application. Rev. Biotechnol. 2018, 38 (3), 321–334. https://doi.org/10.1080/07388551.2017.1354354.
- Mitrofanova, O.; Mardanova, A.; Evtugyn, V.; Bogomolnaya, L.; Sharipova, M. Effects of Bacillus Serine Proteases on the Bacterial Biofilms. BioMed Res. Int. 2017, 2017, 1–10. https://doi.org/10.1155/2017/8525912.
- Hols, P.; Ledesma-García, L.; Gabant, P.; Mignolet, J. Mobilization of Microbiota Commensals and Their Bacteriocins for Therapeutics. Trends Microbiol. 2019, 27 (8), 690–702. https://doi.org/10.1016/j.tim.2019.03.007.
- Preciado, G. M.; EscalanteMinakata, P.; Castro, J. A. O.; Junquera, V. I.; Chávez, J. A. M.; González, C. N. A.; Herrera, R. R. Bacteriocinas: características y aplicación en alimentos. 2013, 8.
- Makhal, S.; Kanawjia, S. K.; Giri, A. Effect of MicroGARD on Keeping Quality of Direct Acidified Cottage Cheese. Food Sci. Technol. 2015, 52 (2), 936–943. https://doi.org/10.1007/s13197-013-1055-2.
- Shin, J. M.; Gwak, J. W.; Kamarajan, P.; Fenno, J. C.; Rickard, A. H.; Kapila, Y. L. Biomedical Applications of Nisin. Appl. Microbiol. 2016, 120 (6), 1449–1465. https://doi.org/10.1111/jam.13033.
- Abanoz, H. S.; Kunduhoglu, B. Antimicrobial Activity of a Bacteriocin Produced by Enterococcus Faecalis KT11 against Some Pathogens and Antibiotic-Resistant Bacteria. Korean J. Food Sci. Anim. Resour. 2018, 38 (5), 1064–1079. https://doi.org/10.5851/kosfa.2018.e40.
- Hasan, F. B.; Reza, M.; Masud, H. A. A.; Uddin, M. K.; Uddin, M. S. Preliminary Characterization and Inhibitory Activity of Bacteriocin like Substances from Lactobacillus Casei against Multi-Drug Resistant Bacteria. Bangladesh J. Microbiol. 2019, 36 (1), 1–6. https://doi.org/10.3329/bjm.v36i1.44259.
- Welman, A. D.; Maddox, I. S. Exopolysaccharides from Lactic Acid Bacteria: Perspectives and Challenges. Trends Biotechnol. 2003, 21 (6), 269–274. https://doi.org/10.1016/S0167-7799(03)00107-0.
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of Lactic Acid Bacteria: Structure, Bioactivity and Associations: A Review. Polym. 2019, 207, 317–332. https://doi.org/10.1016/j.carbpol.2018.11.093.
- Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; Wang, X. Exopolysaccharides from Lactobacillus Buchneri TCP016 Attenuate LPS- and d -GalN-Induced Liver Injury by Modulating the Gut Microbiota. Agric. Food Chem. 2019, 67 (42), 11627–11637. https://doi.org/10.1021/acs.jafc.9b04323.
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus Fermentum S1. Biosci. Bioeng. 2020, 129 (2), 206–214. https://doi.org/10.1016/j.jbiosc.2019.07.009.
- Di, W.; Zhang, L.; Wang, S.; Yi, H.; Han, X.; Fan, R.; Zhang, Y. Physicochemical Characterization and Antitumour Activity of Exopolysaccharides Produced by Lactobacillus Casei SB27 from Yak Milk. Polym. 2017, 171, 307–315. https://doi.org/10.1016/j.carbpol.2017.03.018.
- Guo, Y.; Pan, D.; Li, H.; Sun, Y.; Zeng, X.; Yan, B. Antioxidant and Immunomodulatory Activity of Selenium Exopolysaccharide Produced by Lactococcus Lactis Subsp. Lactis. Food Chem. 2013, 138 (1), 84–89. https://doi.org/10.1016/j.foodchem.2012.10.029.
- Van der Es, D.; Hogendorf, W. F. J.; Overkleeft, H. S.; van der Marel, G. A.; Codée, J. D. C. Teichoic Acids: Synthesis and Applications. Soc. Rev. 2017, 46 (5), 1464–1482. https://doi.org/10.1039/C6CS00270F.
- Brown, S.; Santa Maria, J. P.; Walker, S. Wall Teichoic Acids of Gram-Positive Bacteria. Rev. Microbiol. 2013, 67 (1), 313–336. https://doi.org/10.1146/annurev-micro-092412-155620.
- Lebeer, S.; Claes, I.; Tytgat, H. L. P.; Verhoeven, T. L. A.; Marien, E.; von Ossowski, I.; Reunanen, J.; Palva, A.; de Vos, W. M.; De Keersmaecker, S. C. J.; Vanderleyden, J. Functional Analysis of Lactobacillus Rhamnosus GG Pili in Relation to Adhesion and Immunomodulatory Interactions with Intestinal Epithelial Cells. Environ. Microbiol. 2012, 78 (1), 185–193. https://doi.org/10.1128/AEM.06192-11.
- Kim, K. W.; Kang, S.-S.; Woo, S.-J.; Park, O.-J.; Ahn, K. B.; Song, K.-D.; Lee, H.-K.; Yun, C.-H.; Han, S. H. Lipoteichoic Acid of Probiotic Lactobacillus Plantarum Attenuates Poly I:C-Induced IL-8 Production in Porcine Intestinal Epithelial Cells. Microbiol. 2017, 8, 1827. https://doi.org/10.3389/fmicb.2017.01827.
- Claes, I. J.; Segers, M. E.; Verhoeven, T. L.; Dusselier, M.; Sels, B. F.; De Keersmaecker, S. C.; Vanderleyden, J.; Lebeer, S. Lipoteichoic Acid Is an Important Microbe-Associated Molecular Pattern of Lactobacillus Rhamnosus GG. Cell Factories 2012, 11 (1), 161. https://doi.org/10.1186/1475-2859-11-161.
- Ahn, J. E.; Kim, H.; Chung, D. K. Lipoteichoic Acid Isolated from Lactobacillus Plantarum Maintains Inflammatory Homeostasis through Regulation of Th1- and Th2- Induced Cytokines. Microbiol. Biotechnol. 2019, 29 (1), 151–159. https://doi.org/10.4014/jmb.1809.09001.
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S. P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D. A.; Kritchevsky, S. B.; Kitzman, D. W.; Yadav, H. Lipoteichoic Acid from the Cell Wall of a Heat Killed Lactobacillus Paracasei D3-5 Ameliorates Aging-Related Leaky Gut, Inflammation and Improves Physical and Cognitive Functions: From C. Elegans to Mice. GeroScience 2020, 42 (1), 333–352. https://doi.org/10.1007/s11357-019-00137-4.
- LeBlanc, J. G.; Laiño, J. E.; del Valle, M. J.; de Giori, G. S.; Sesma, F.; Taranto, M. P. B-Group Vitamins Production by Probiotic Lactic Acid Bacteria. In Biotechnology of Lactic Acid Bacteria; Mozzi, F., Raya, R. R., Vignolo, G. M., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2015; pp 279–296. https://doi.org/10.1002/9781118868386.ch17.
- Deptula, P.; Chamlagain, B.; Edelmann, M.; Sangsuwan, P.; Nyman, T. A.; Savijoki, K.; Piironen, V.; Varmanen, P. Food-Like Growth Conditions Support Production of Active Vitamin B12 by Propionibacterium Freudenreichii 2067 without DMBI, the Lower Ligand Base, or Cobalt Supplementation. Microbiol. 2017, 8. https://doi.org/10.3389/fmicb.2017.00368.
- Thakur, K.; Lule, V.; Kumar, N.; Mandal, S.; Anand, S.; Kumari, V.; Tomar, S. Riboflavin Producing Probiotic Lactobacilli as a Biotechnological Strategy to Obtain Riboflavin-Enriched Fermented Foods. Pure Appl. Microbiol. 2016, 10, 161–166.
- Bardosono, S.; Wibowo, N.; Sutanto, L. B.; Irwinda, R.; Cannan, R.; Rowan, A.; Dekker, J. Plasma Folate, Vitamin B6 and B12 in Their Relationship to the Presence of Probiotic Strain Bifidobacterium Animalis Subsp. Lactis HNO19 (DR10TM) Among Indonesian Pregnant Women in Their Third Semester. World Nutr. J. 2019, 2 (2), 56. https://doi.org/10.25220/WNJ.V02.i2.0009.
- Satpute, S. K.; Kulkarni, G. R.; Banpurkar, A. G.; Banat, I. M.; Mone, N. S.; Patil, R. H.; Cameotra, S. S. Biosurfactant/s from Lactobacilli Species: Properties, Challenges and Potential Biomedical Applications: Biosurfactant/s from Lactobacilli Species. Basic Microbiol. 2016, 56 (11), 1140–1158. https://doi.org/10.1002/jobm.201600143.
- Morais, I. M. C.; Cordeiro, A. L.; Teixeira, G. S.; Domingues, V. S.; Nardi, R. M. D.; Monteiro, A. S.; Alves, R. J.; Siqueira, E. P.; Santos, V. L. Biological and Physicochemical Properties of Biosurfactants Produced by Lactobacillus Jensenii P6A and Lactobacillus Gasseri P65. Cell Factories 2017, 16 (1), 155. https://doi.org/10.1186/s12934-017-0769-7.
- Merghni, A.; Dallel, I.; Noumi, E.; Kadmi, Y.; Hentati, H.; Tobji, S.; Ben Amor, A.; Mastouri, M. Antioxidant and Antiproliferative Potential of Biosurfactants Isolated from Lactobacillus Casei and Their Anti-Biofilm Effect in Oral Staphylococcus Aureus Strains. Pathog. 2017, 104, 84–89. https://doi.org/10.1016/j.micpath.2017.01.017.
- Fracchia, L.; J. Banat, J.; Cavallo, M.; Ceresa, C.; M. Banat, I.; 1 Department of Pharmaceutical Sciences, Università del Piemonte Orientale “A. Avogadro”, Largo Donegani 2, 28100, Novara, Italy; Potential Therapeutic Applications of Microbial Surface-Active Compounds. AIMS Bioeng. 2015, 2 (3), 144–162. https://doi.org/10.3934/bioeng.2015.3.144
