Ubiquitously present in plant life, coumarins have multiple applications—in everyday life, in organic synthesis, in medicine and many others. They are well known for their broad spectrum of physiological effects. The specific structure of the coumarin scaffold involves a conjugated system with excellent charge and electron transport properties. The antioxidant activity of natural coumarins has been a subject of intense study for at least two decades. Significant research into the antioxidant behavior of natural/semi-synthetic coumarins and their complexes has been carried out and published in scientific literature.
- antioxidant
- coumarins
- radicals’ scavenging
- antioxidant assays
1. Introduction
| Source of RS | Type of RS | Site of Production | Function |
|---|---|---|---|
| Nicotinamide adenine dinucleotide (NADH) dehydrogenase (Complex I) | superoxide | Mitochondria | Electron transport during oxidative phosphorylation. |
| Coenzyme Q (Complex III) | superoxide | Mitochondria | Electron transport during oxidative phosphorylation. |
| Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) | superoxide | Cytosol, membranes of various organelles | Immune response, phagocytosis. |
| Xanthine oxidase | Superoxide, hydrogen peroxide | Liver, intestines | Catalytic conversion of hypoxanthine to xanthine and uric acid. |
| Nitric oxide synthase | Nitric oxide (NO), superoxide | Cytosol, cellular membranes | Synthesis of NO from L-arginine. |
| Myeloperoxidase | Hypochlorous acid | Neutrophils | Synthesis of hypochlorous acid during respiratory burst. |
| 5-lipoxygenase | Indirect action | Immune cells | Leukotriene production, causing NOX stimulation and RS generation. |
| Peroxynitrite production | Peroxynitrite ion | Phagocytes | Produced from superoxide and NO, phagocytosis. |
| Fenton reaction and Haber–Weiss chain | Hydroxyl/hydroperoxyl radicals | Wherever transition metal ions such as iron and copper come in contact with hydrogen peroxide and/or superoxide. | Mostly associated with pathologies. |
2. Antioxidant Properties of Molecular Coumarins
2.1. Coumarins Substituted with Small Functionalities
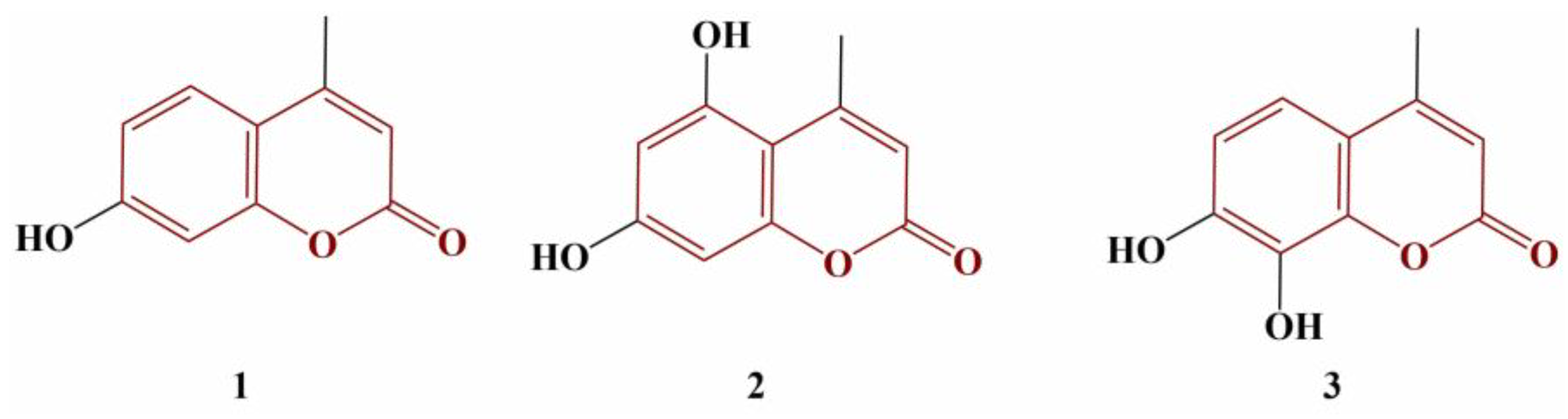
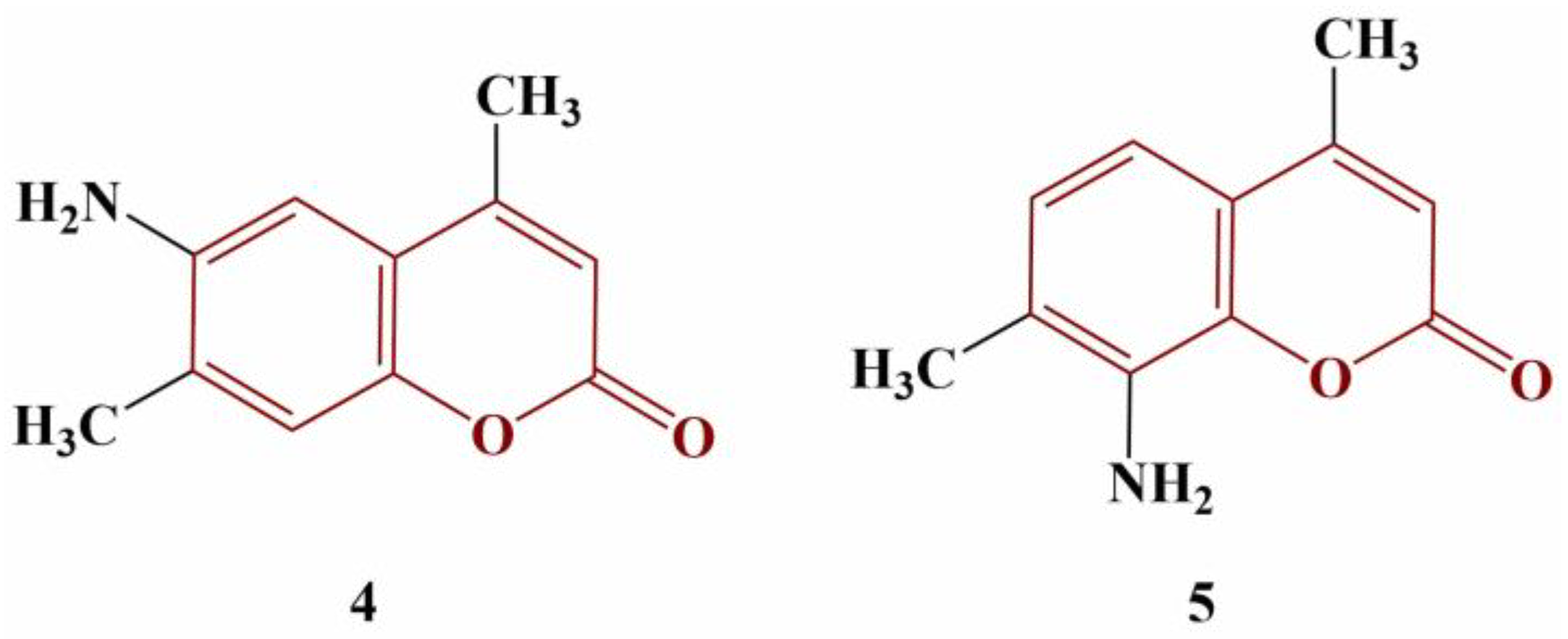
2.2. Coumarins, Substituted at Positions 3 and/or 4
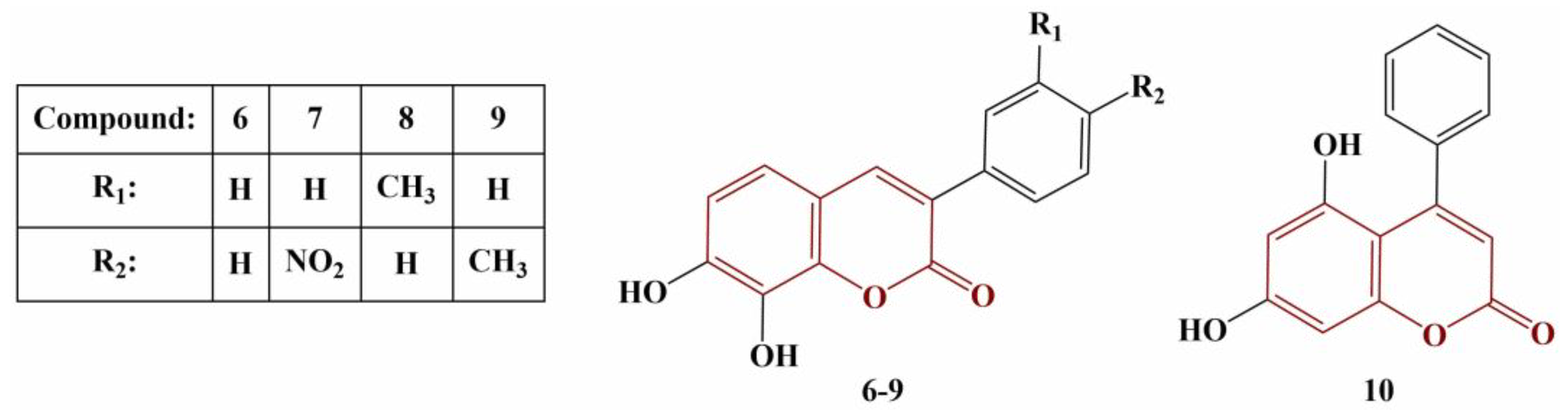
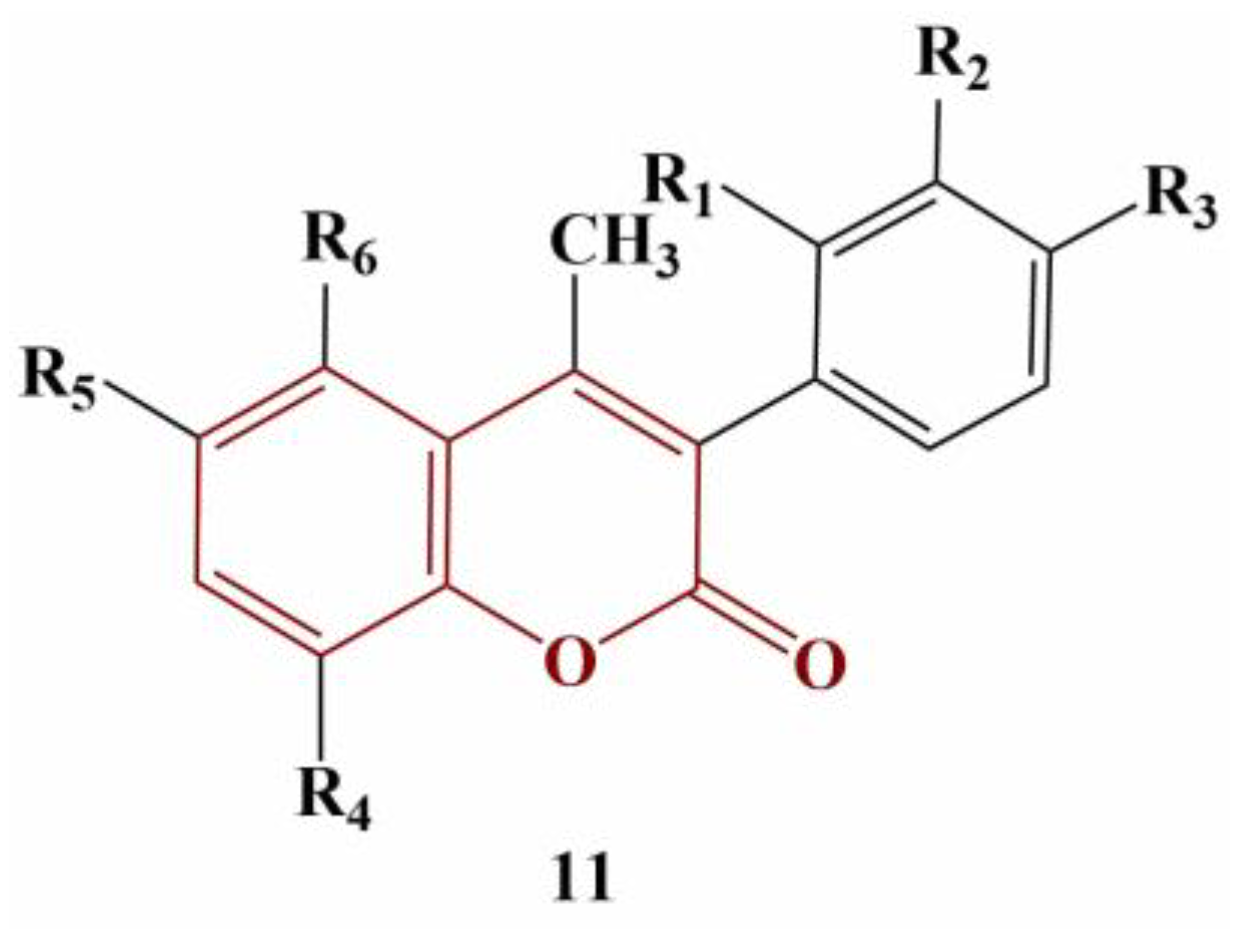
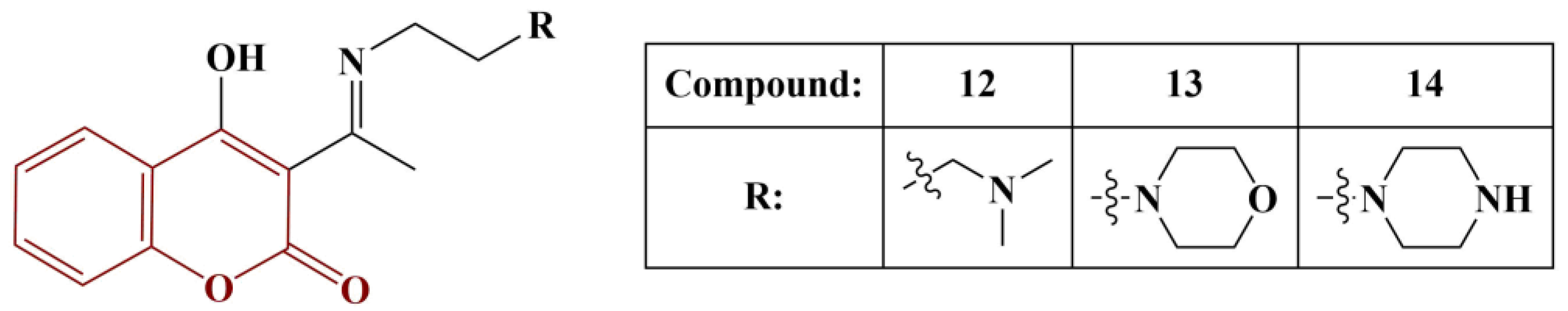
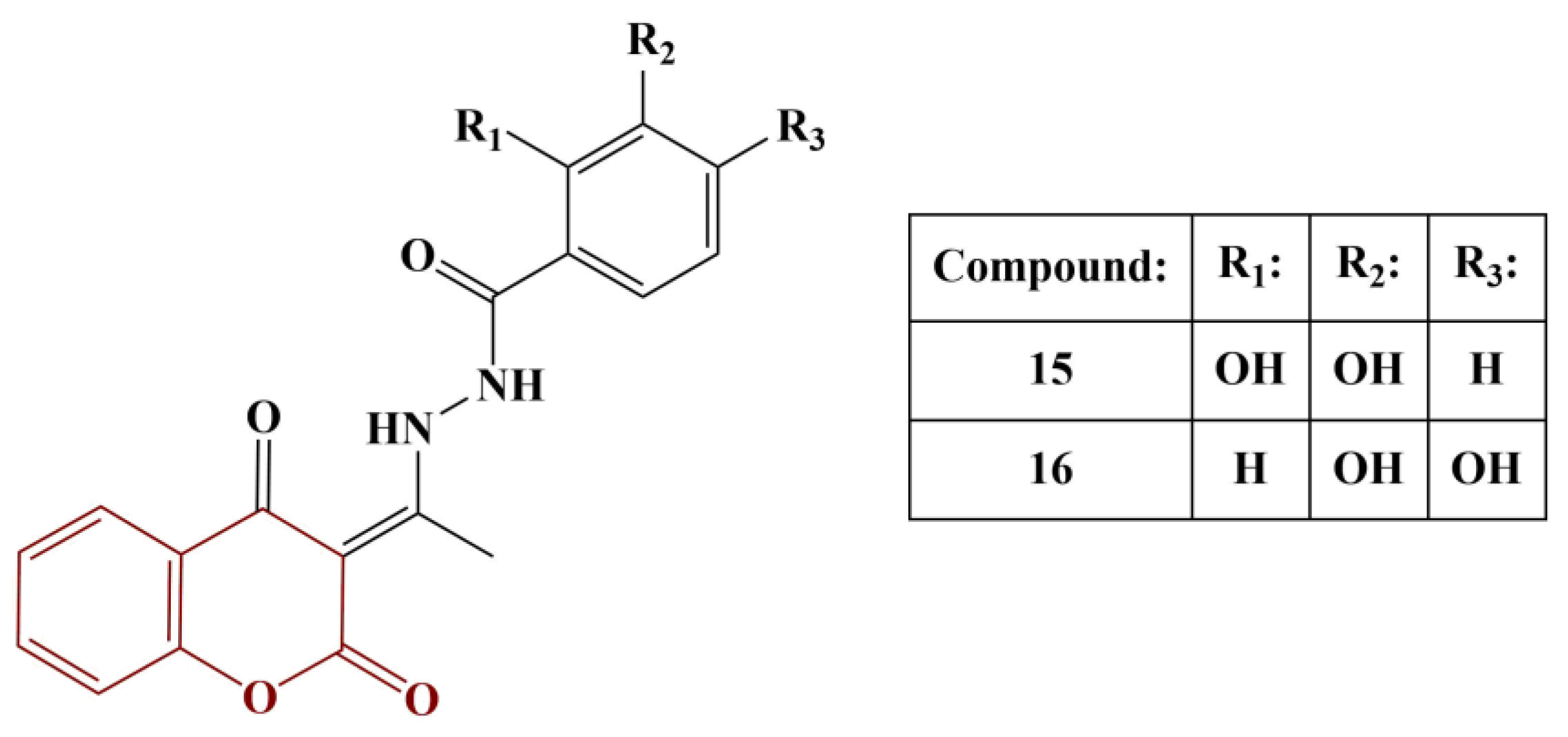
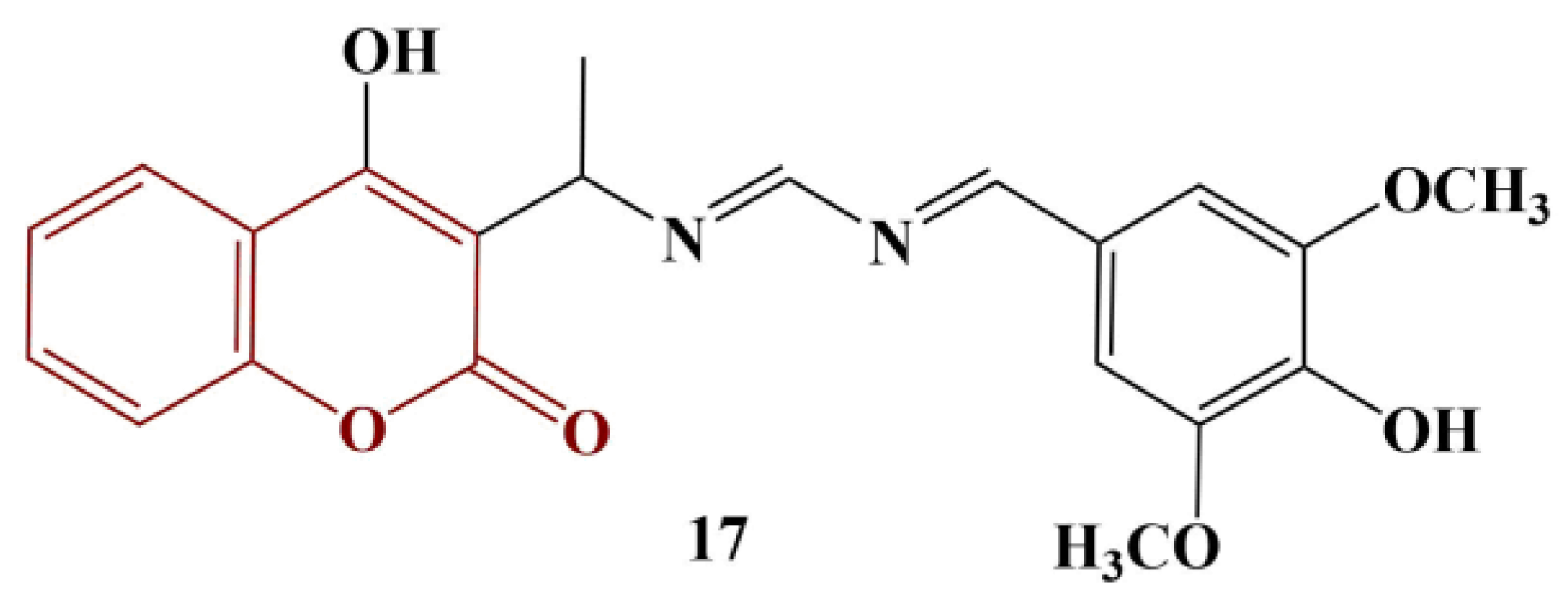
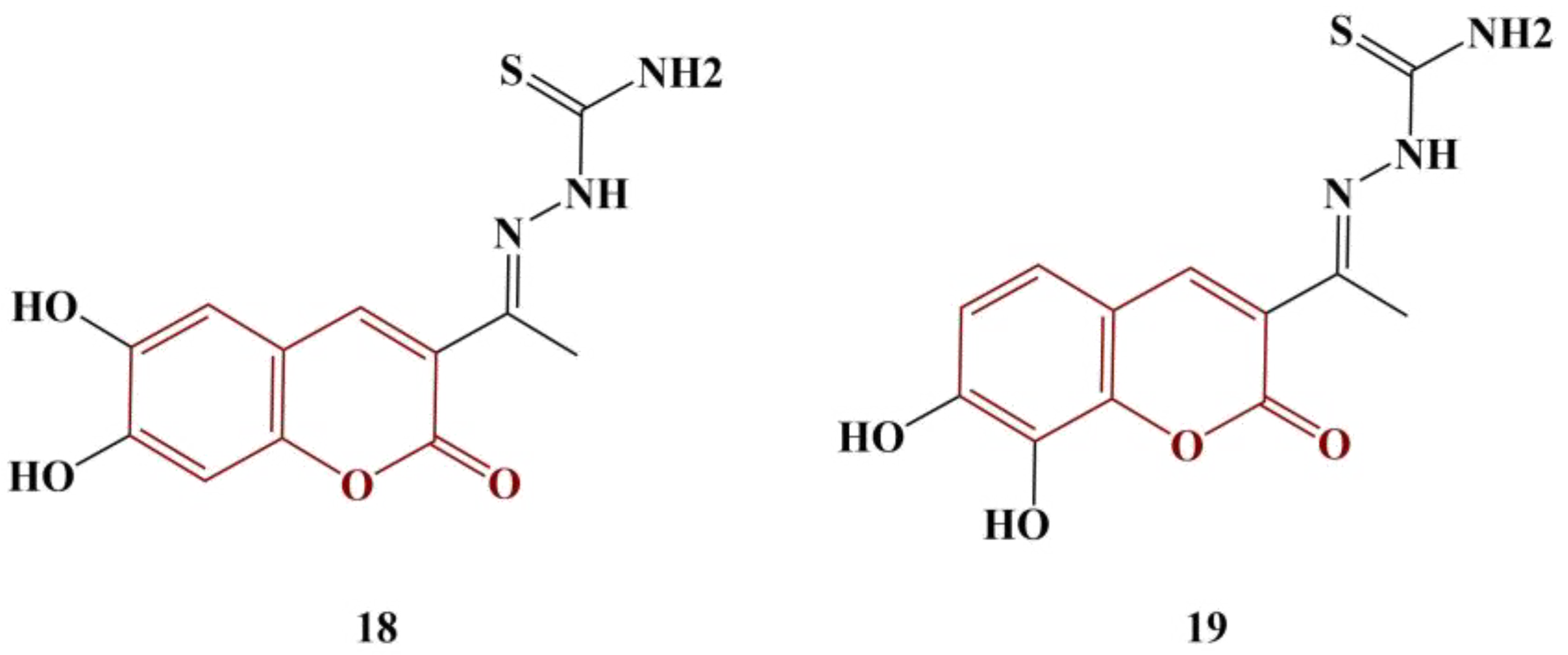
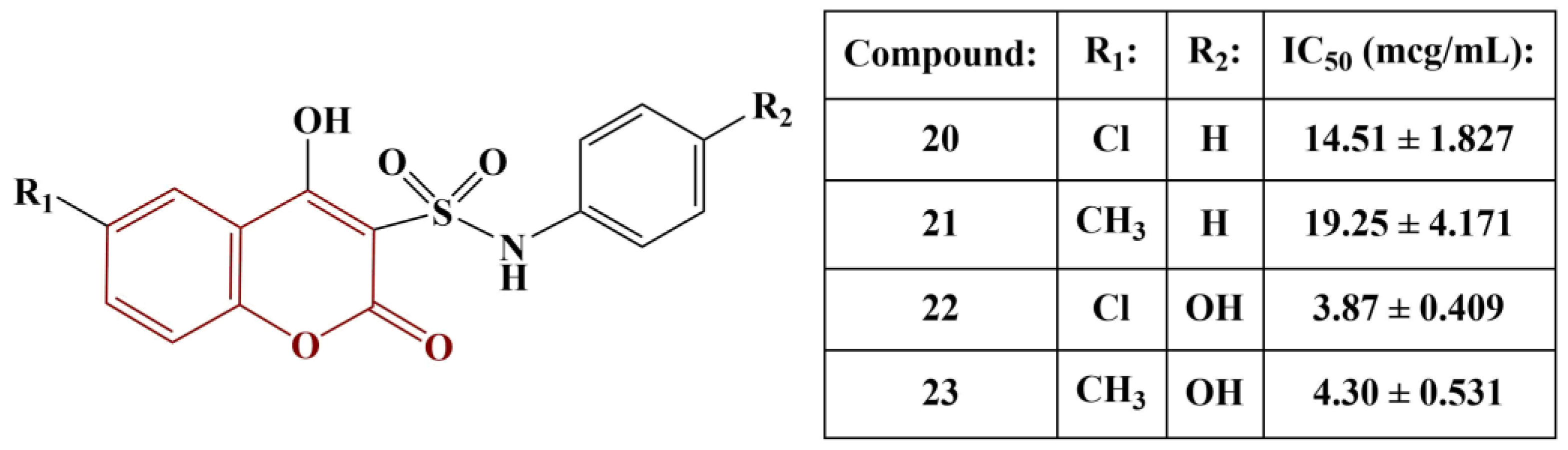
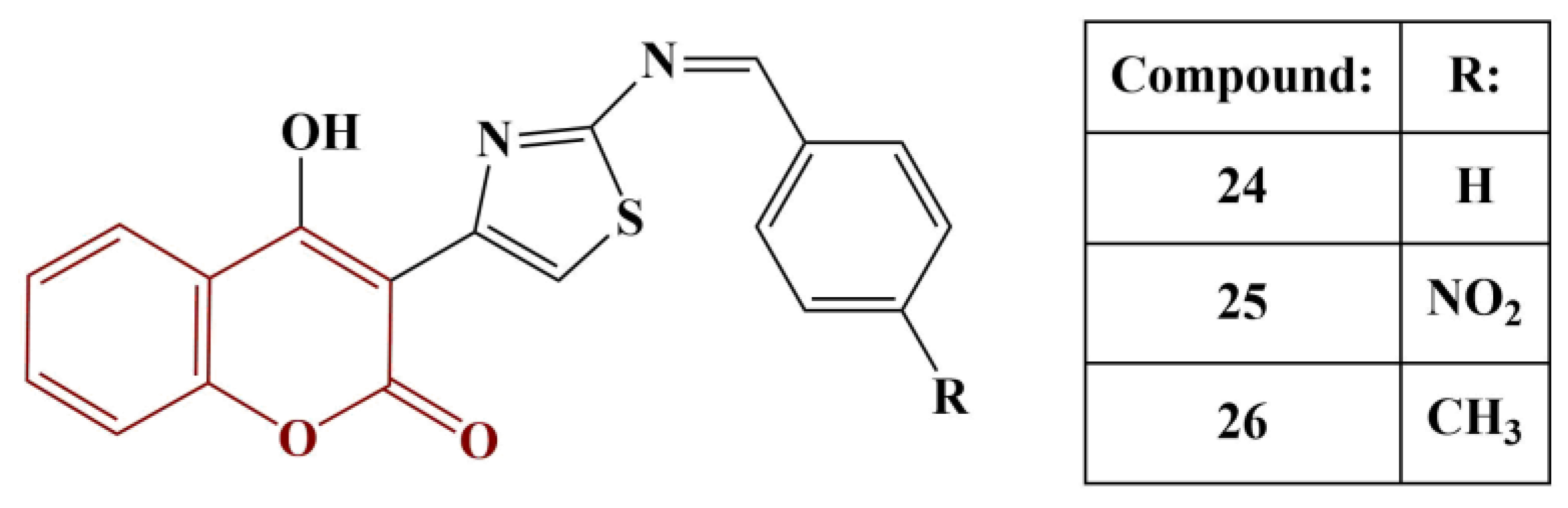
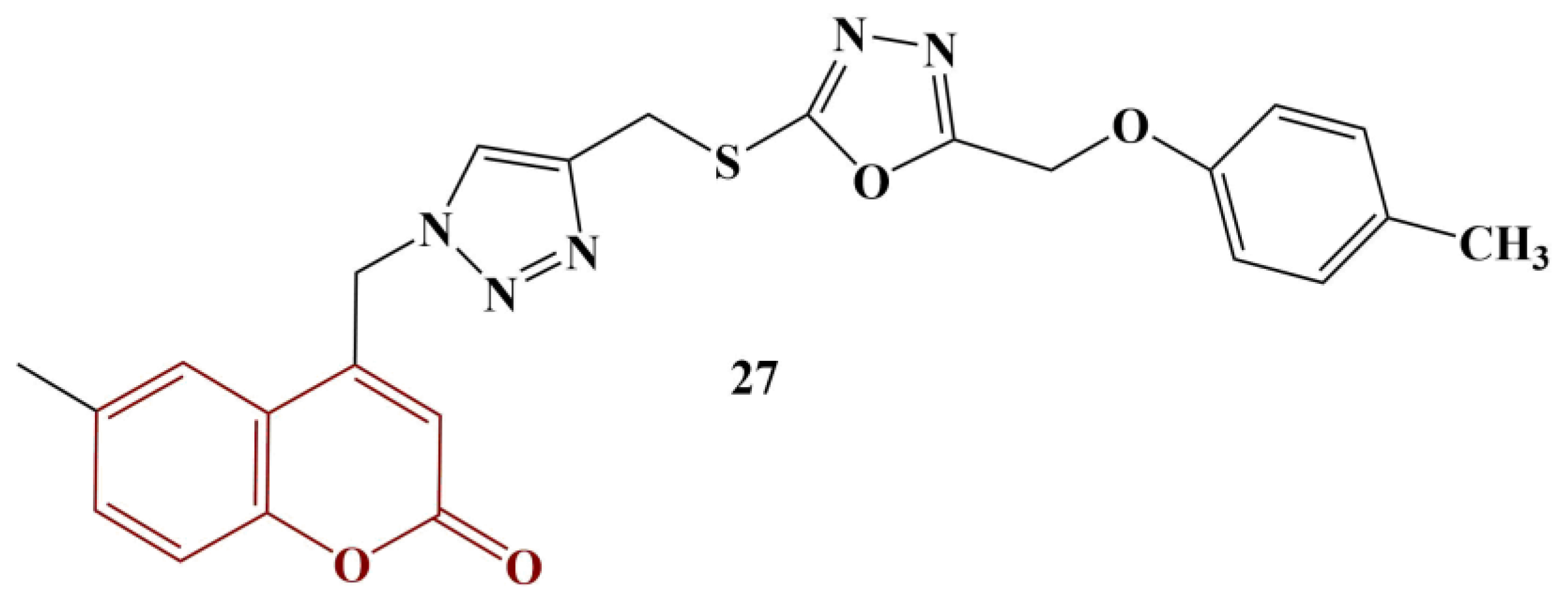
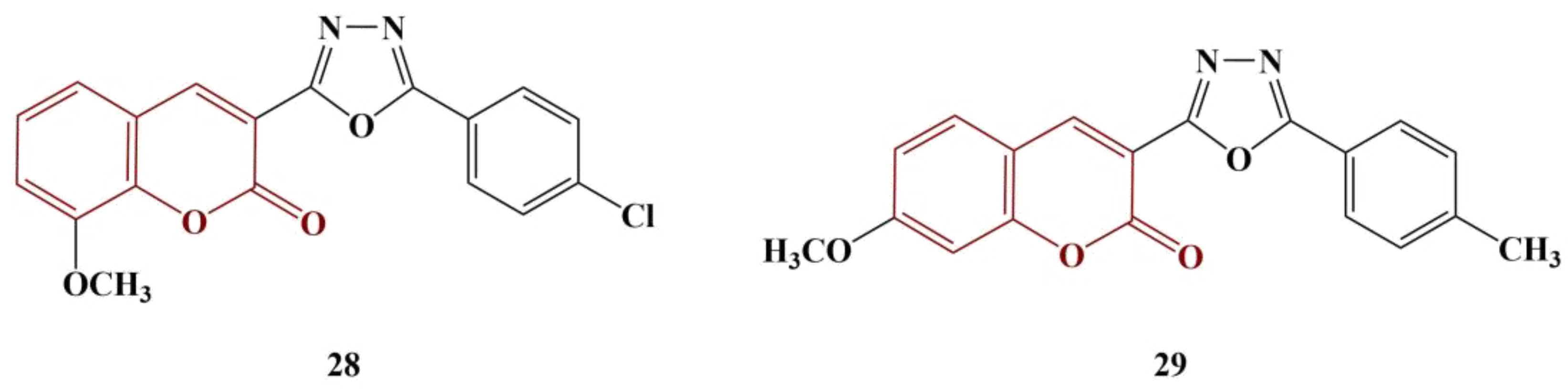
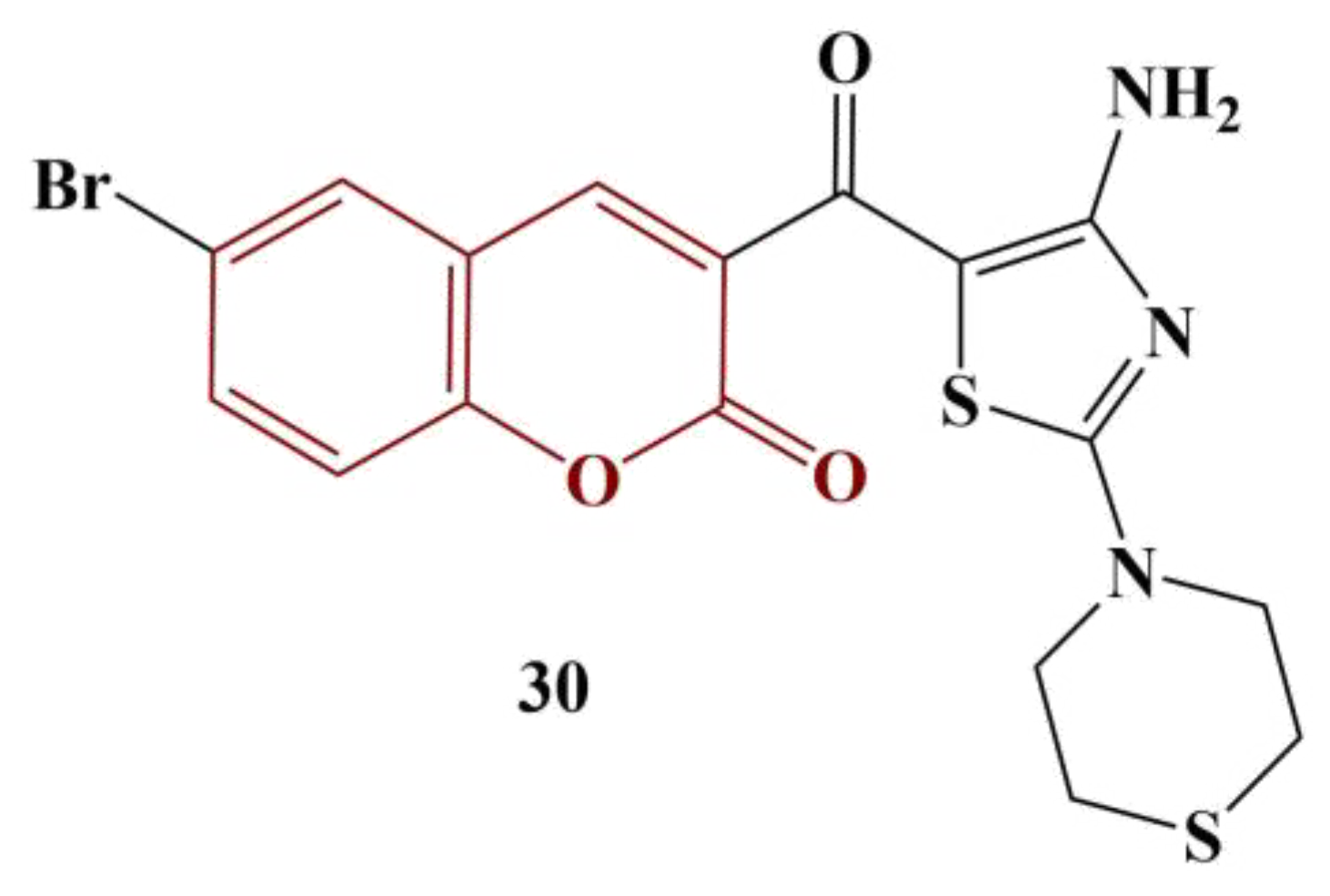
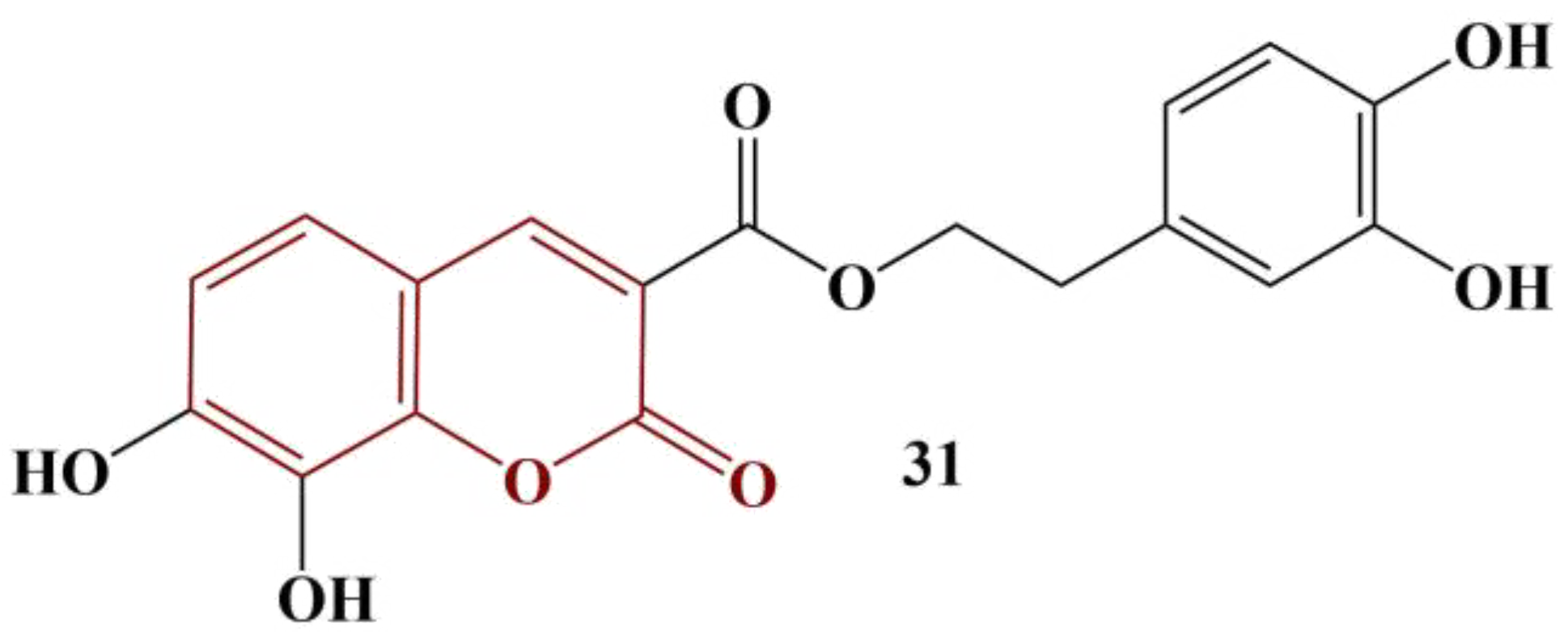
2.3. Coumarins, Substituted at Positions 7 and/or 8
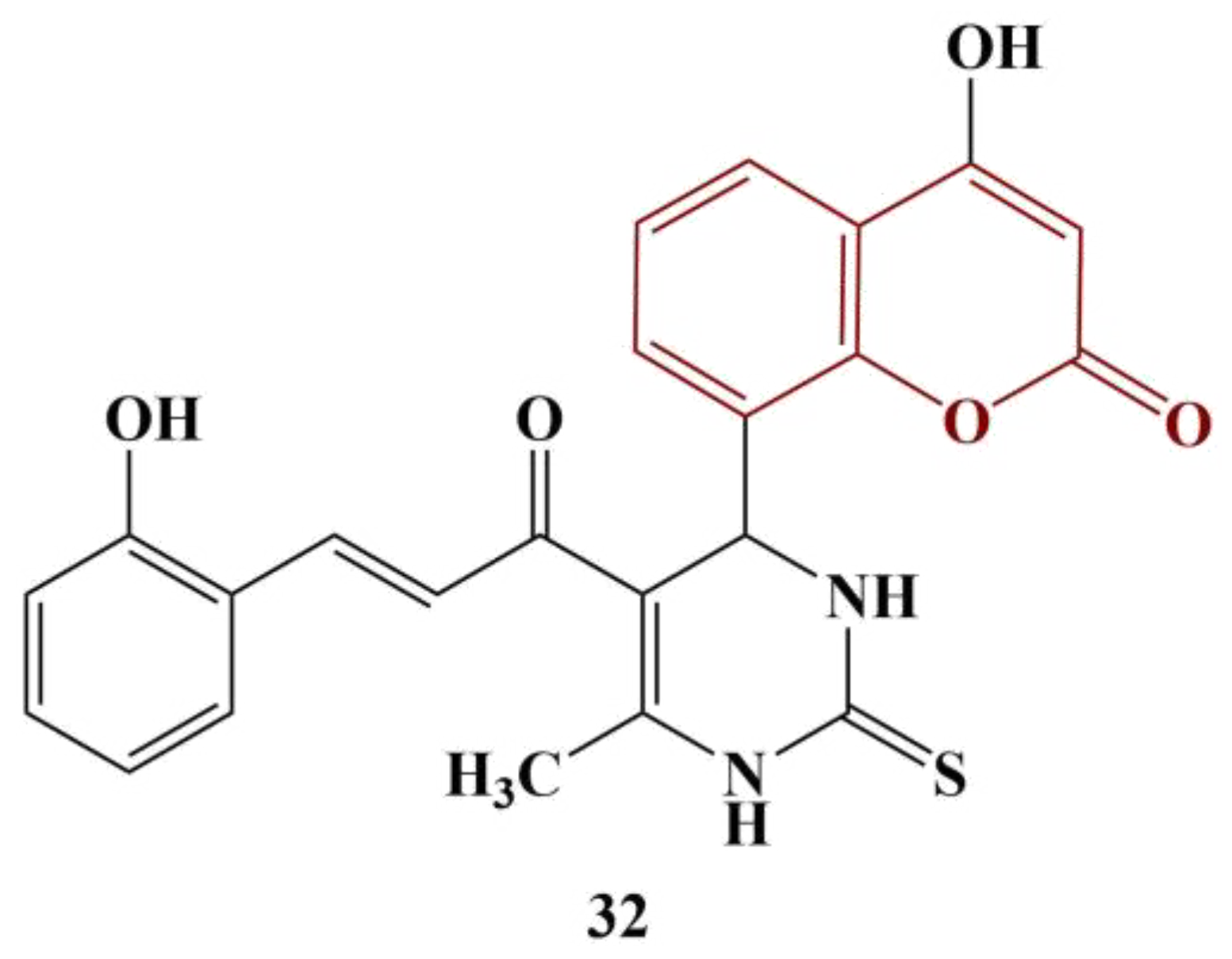
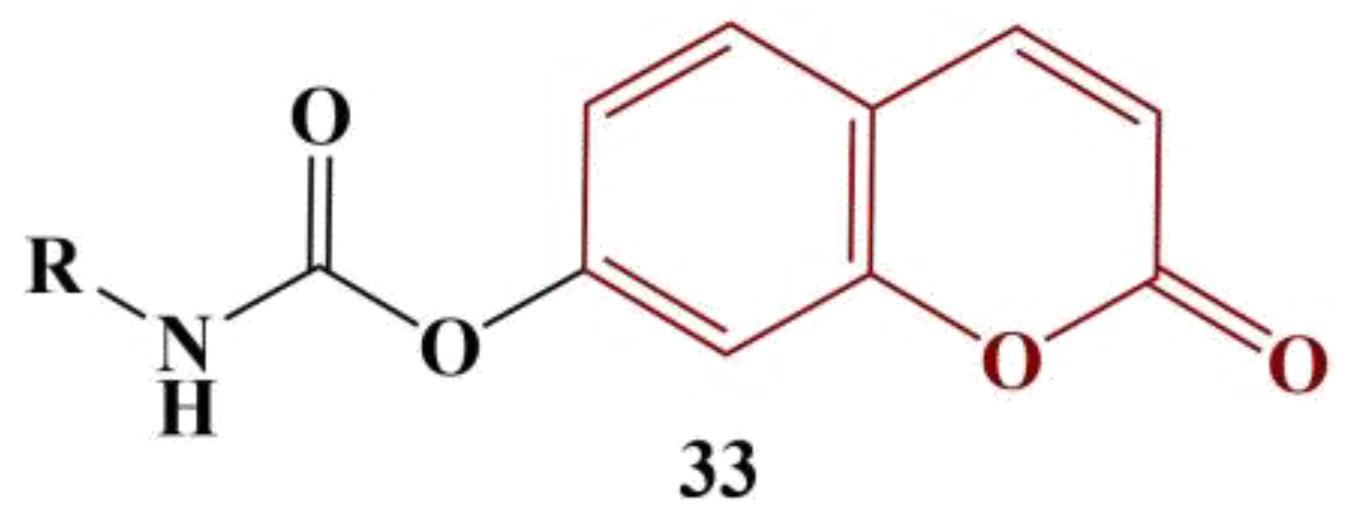
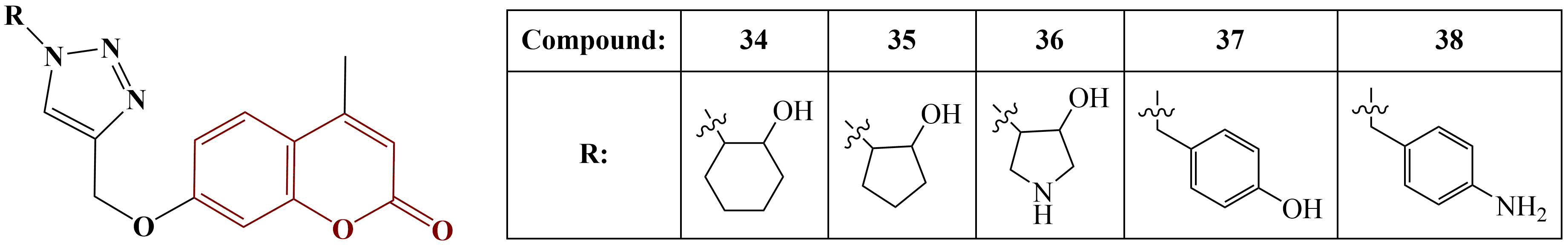
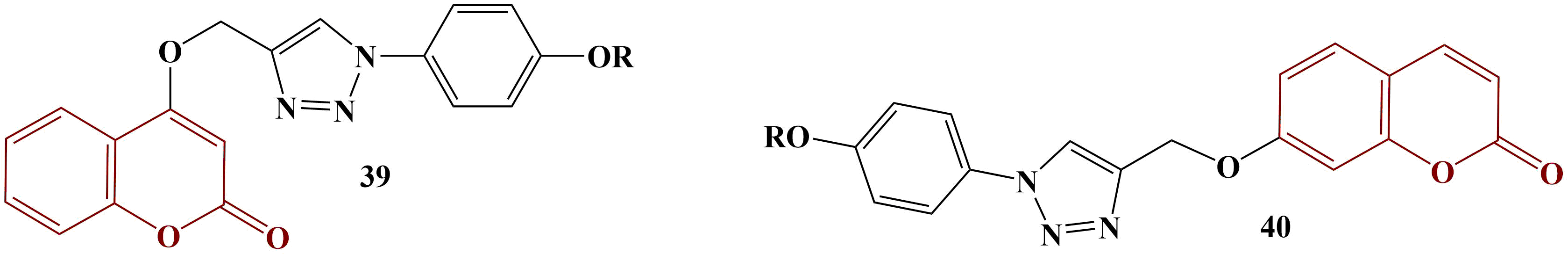
On the other hand, 4-F and 4-CH3 substitution provided the best result with the ABTS test (IC50= 53.92 and 52.00 μg/mL respectively, ascorbic acid had IC50= 22.64 μg/mL). Contrary to DPPH, 2-Cl, 4-Cl and hydrogen substitution in the benzene ring decreases ABTS scavenging.
Xue and coworkers performed theoretical investigations of a number of 7-hydroxycoumarin – chalcone hybrids[45]. The chalcone moiety was attached at position 8 of the coumarin structure. The results obtained demonstrated that the 7-OH group in ring B of the coumarin structure is less favorable as a donor of hydrogen atom compared to differently positioned OH groups in the same ring. Theoretical calculations demonstrated that the position 7 hydroxyl group forms an intramolecular hydrogen bond with the chalcone carbonyl oxygen atom. The presence of such a bond increases the bond dissociation energy of the 7-OH and makes it less favourable for participation in HAT, compared to OH groups at the chalcone benzene ring. In gas phase/benzene medium the HAT mechanism was calculated to be thermodynamically more favourable.
Gunduz and coworkers synthesized a new coumarin derivative - 7-((8-(4-benzylpiperidin-1-yl)octyl)oxy)-4-methyl-2H-chromen-2-one[46]. The novel compound was tested for antiproliferative and antioxidant activity. DPPH assay showed moderate activity (concentrations between 0.03125 and 1.0 mg/mL), weaker than that of the standard BHT at the same concentrations. Polar media (ethanol or water) seemed to favor the SPLET mechanism, as solvation was calculated to decrease proton affinities.
This entry is adapted from the peer-reviewed paper 10.3390/ph16050651
References
- Dowling, D.K.; Simmons, L.W. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B Biol. Sci. 2009, 276, 1737–1745.
- Linnane, A.W.; Eastwood, H. Cellular redox regulation and prooxidant signaling systems: A new perspective on the free radical theory of aging. Ann. N. Y. Acad. Sci. 2006, 1067, 47–55.
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64.
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119.
- Simpson, D.S.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743.
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species: A key hallmark of cardiovascular disease. Adv. Med. 2016, 2016, 9152732.
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875.
- Filipsky, T.; Riha, M.; Macakova, K.; Anzenbacherová, E.; Karlickova, J.; Mladenka, P. Antioxidant effects of coumarins include direct radical scavenging, metal chelation and inhibition of ROS-producing enzymes. Curr. Top. Med. Chem. 2015, 15, 415–431.
- Sharifi-Rad, J.; Cruz-Martins, N.; López-Jornet, P.; Lopez, E.P.-F.; Harun, N.; Yeskaliyeva, B.; Beyatli, A.; Sytar, O.; Shaheen, S.; Sharopov, F. Natural coumarins: Exploring the pharmacological complexity and underlying molecular mechanisms. Oxidative Med. Cell. Longev. 2021, 2021, 6492346.
- Pedersen, J.Z.; Oliveira, C.; Incerpi, S.; Kumar, V.; Fiore, A.M.; De Vito, P.; Prasad, A.K.; Malhotra, S.V.; Parmar, V.S.; Saso, L. Antioxidant activity of 4-methylcoumarins. J. Pharm. Pharmacol. 2007, 59, 1721–1728.
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334.
- Payá, M.; Halliwell, B.; Hoult, J. Interactions of a series of coumarins with reactive oxygen species: Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 1992, 44, 205–214.
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951.
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833.
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2017, 8, 24.
- Prahadeesh, N.; Sithambaresan, M.; Mathiventhan, U. A study on hydrogen peroxide scavenging activity and ferric reducing ability of simple coumarins. Emerg. Sci. J. 2018, 2, 417–427.
- Couttolenc, A.; Díaz-Porras, Á.; Espinoza, C.; Medina, M.E.; Trigos, Á. On the primary and secondary antioxidant activity from hydroxy-methylcoumarins: Experimental and theoretical studies. J. Phys. Org. Chem. 2020, 33, e4025.
- Smolyaninov, I.; Kuzmin, V.; Arsenyev, M.; Smolyaninova, S.; Poddel’sky, A.; Berberova, N. Electrochemical transformations and anti/prooxidant activity of sterically hindered o-benzoquinones. Russ. Chem. Bull. 2017, 66, 1217–1229.
- Mohammed, A.Y.; Ahamed, L.S. Synthesis and Characterization of New Substituted Coumarin Derivatives and Study Their Biological Activity. Chem. Methodol. 2022, 6, 813–822.
- Ozalp, L.; Danış, Ö.; Yuce-Dursun, B.; Demir, S.; Gündüz, C.; Ogan, A. Investigation of HMG-CoA reductase inhibitory and antioxidant effects of various hydroxycoumarin derivatives. Arch. Pharm. 2020, 353, 1900378.
- Katopodi, A.; Tsotsou, E.; Iliou, T.; Deligiannidou, G.-E.; Pontiki, E.; Kontogiorgis, C.; Tsopelas, F.; Detsi, A. Synthesis, bioactivity, pharmacokinetic and biomimetic properties of multi-substituted coumarin derivatives. Molecules 2021, 26, 5999.
- Yang, A.; Zhang, H.; Hu, C.; Wang, X.; Shen, R.; Kou, X.; Wang, H. Novel coumarin derivatives as multifunctional anti-AD agents: Design, synthesis, X-ray crystal structure and biological evaluation. J. Mol. Struct. 2022, 1268, 133747.
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green one-pot synthesis of coumarin-hydroxybenzohydrazide hybrids and their antioxidant potency. Antioxidants 2021, 10, 1106.
- Ristić, M.N.; Radulović, N.S.; Dekić, B.R.; Dekić, V.S.; Ristić, N.R.; Stojanović-Radić, Z. Synthesis and spectral characterization of asymmetric azines containing a coumarin moiety: The discovery of new antimicrobial and antioxidant agents. Chem. Biodivers. 2019, 16, e1800486.
- Masuri, S.; Era, B.; Pintus, F.; Cadoni, E.; Cabiddu, M.G.; Fais, A.; Pivetta, T. Hydroxylated Coumarin-Based Thiosemicarbazones as Dual Antityrosinase and Antioxidant Agents. Int. J. Mol. Sci. 2023, 24, 1678.
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.; Mahmoud, A.H.; Ibrahim, B.M.; Bari, A.; Villinger, A. Synthesis and evaluation of new coumarin derivatives as antioxidant, antimicrobial, and anti-inflammatory agents. Molecules 2020, 25, 3251.
- Bensalah, D.; Mnasri, A.; Chakchouk-Mtibaa, A.; Mansour, L.; Mellouli, L.; Hamdi, N. Synthesis and antioxidant properties of some new thiazolyl coumarin derivatives. Green Chem. Lett. Rev. 2020, 13, 155–163.
- Kumar, K.A.; Kalluraya, B.; Kumar, S.M. Synthesis and in-vitro antioxidant activities of some coumarin derivatives containing 1, 2, 3-triazole ring. Phosphorus Sulfur Silicon Relat. Elem. 2018, 193, 294–299.
- Naik, M.D.; Bodke, Y.D.; BC, R. An efficient one-pot synthesis of coumarin-amino acid derivatives as potential anti-inflammatory and antioxidant agents. Synth. Commun. 2020, 50, 1210–1216.
- Abd-Almonuim, A.E.; Mohammed, S.M.; Al-Khalifa, I.I. Preparation, Characterization and Antioxidant Determination of Coumarin Substituted Heterocyclic Compound. Asian J. Res. Chem. 2020, 13, 23–27.
- Basappa, V.C.; Penubolu, S.; Achutha, D.K.; Kariyappa, A.K. Synthesis, characterization and antioxidant activity studies of new coumarin tethered 1, 3, 4-oxadiazole analogues. J. Chem. Sci. 2021, 133, 55.
- Ayati, A.; Bakhshaiesh, T.O.; Moghimi, S.; Esmaeili, R.; Majidzadeh-A, K.; Safavi, M.; Firoozpour, L.; Emami, S.; Foroumadi, A. Synthesis and biological evaluation of new coumarins bearing 2,4-diaminothiazole-5-carbonyl moiety. Eur. J. Med. Chem. 2018, 155, 483–491.
- Li, W.-B.; Qiao, X.-P.; Wang, Z.-X.; Wang, S.; Chen, S.-W. Synthesis and antioxidant activity of conjugates of hydroxytyrosol and coumarin. Bioorg. Chem. 2020, 105, 104427.
- Konidala, S.K.; Kotra, V.; Danduga, R.C.S.R.; Kola, P.K.; Bhandare, R.R.; Shaik, A.B. Design, multistep synthesis and in-vitro antimicrobial and antioxidant screening of coumarin clubbed chalcone hybrids through molecular hybridization approach. Arab. J. Chem. 2021, 14, 103154.
- De Souza, G.A.; da Silva, S.J.; Del Cistia, C.d.N.; Pitasse-Santos, P.; Pires, L.d.O.; Passos, Y.M.; Cordeiro, Y.; Cardoso, C.M.; Castro, R.N.; Sant’Anna, C.M.R. Discovery of novel dual-active 3-(4-(dimethylamino) phenyl)-7-aminoalcoxy-coumarin as potent and selective acetylcholinesterase inhibitor and antioxidant. J. Enzyme Inhib. Med. Chem. 2019, 34, 631–637.
- Matos, M.J.; Herrera Ibata, D.M.; Uriarte, E.; Viña, D. Coumarin-Rasagiline Hybrids as Potent and Selective hMAO-B Inhibitors, Antioxidants, and Neuroprotective Agents. ChemMedChem 2020, 15, 532–538.
- Joy, M.; Bodke, Y.; Telkar, S. 4-Methyl-7-alkynyl coumarin derivatives as potent antimicrobials and antioxidants. Bulg. Chem. Commun. 2020, 52, 237–244.
- Kurt, B.Z.; Gazioglu, I.; Kandas, N.O.; Sonmez, F. Synthesis, anticholinesterase, antioxidant, and anti-aflatoxigenic activity of novel coumarin carbamate derivatives. ChemistrySelect 2018, 3, 3978–3983.
- Nibin Joy, M.; Bodke, Y.D.; Telkar, S.; Bakulev, V.A. Synthesis of coumarins linked with 1, 2, 3-triazoles under microwave irradiation and evaluation of their antimicrobial and antioxidant activity. J. Mex. Chem. Soc. 2020, 64, 53–73.
- Popova, S.A.; Shevchenko, O.G.; Chukicheva, I.Y. Synthesis of new coumarin oxazine derivatives of 7-hydroxy-6-isobornyl-4-methylcoumarin and their antioxidant activity. Chem. Biol. Drug Des. 2022, 100, 994–1004.
- Kaushik, C.; Chahal, M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1, 4-disubstituted 1, 2, 3-triazoles. Mon. Chem. Chem. Mon. 2021, 152, 1001–1012.
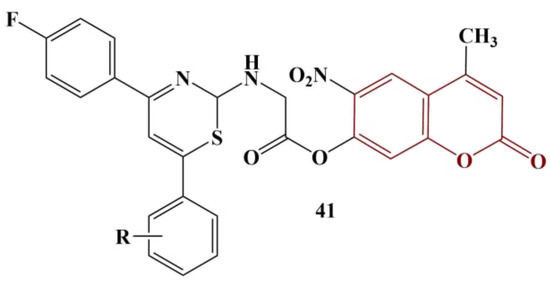
- Chauhan, N.B.; Patel, N.B.; Patel, V.M.; Mistry, B.M. Synthesis and biological evaluation of coumarin clubbed thiazines scaffolds as antimicrobial and antioxidant. Med. Chem. Res. 2018, 27, 2141–2149.
- Xue, Y.; Liu, Y.; Luo, Q.; Wang, H.; Chen, R.; Liu, Y.; Li, Y. Antiradical activity and mechanism of coumarin–chalcone hybrids: Theoretical insights. The Journal of Physical Chemistry A 2018, 122, 8520-8529.
- Kecel-Gunduz, S.; Budama-Kilinc, Y.; Bicak, B.; Gok, B.; Belmen, B.; Aydogan, F.; Yolacan, C. New coumarin derivative with potential antioxidant activity: Synthesis, DNA binding and in silico studies (Docking, MD, ADMET). Arabian Journal of Chemistry 2023, 16, 104440.
