Atherosclerosis is a progressive vascular multifactorial process. The mechanisms underlining the initiating event of atheromatous plaque formation are inflammation and oxidation, with Platelet-Activating Factor (PAF), a potent lipid inflammatory mediator, implicated in the initiation and propagation of atherosclerosis according to a new theory “The PAF implicated atherosclerosis theory” [8]. This theory also proposes an explanation of the beneficial role of the Mediterranean diet (MedDiet) against atherosclerosis and cardiovascular diseases, in a biochemical approach (see https://youtu.be/yKrWBA7l7s4). Among the modifiable risk factors for cardiovascular diseases, diet and especially the MedDiet, has been widely recognized as one of the healthiest dietary patterns. Olive oil (OO), the main source of the fatty components of the MedDiet is superior to the other “Mono-unsaturated fatty acids containing oils” due to the existence of specific microconstituents.
- atherosclerosis
- cardiovascular diseases
- olive oil
- olive oil by-products
- atherosclerosis cardiovascular diseases olive oil olive oil by-products Platelet Activating Factor. PAF
- Platelet Activating Factor, PAF
1. Olive Oil
From several epidemiological and clinical studies, it has been documented that OO, and especially virgin olive oil (VOO) intake, has many beneficial effects in atherosclerosis and in the majority of CVDs. Briefly, the intake of OO results in reduced blood pressure, improved blood lipid metabolism, by reducing triacylglycerols and LDL oxidation, while increasing HDL levels, improved plasma glucose, and HbA1c as well as post-prandial glucose levels and contributes to an enhanced endothelial function. OO exerts anti-inflammatory, antioxidant, and anti-thrombotic actions [38]. Recent meta-analyses and review articles suggest that OO microconstituents are mainly responsible for its antiatherogenic properties, either alone or in synergy [39,40]. Therefore, this review focuses on data from in vitro experiments, from studies conducted with animal models as well as from human studies that were carried out mainly with discrete and separated OO microconstituents in an attempt to highlight those that play a determinant role in modulating atherosclerosis development, with special attention on microconstituents with inhibitory activity against PAF actions.
The basic composition of OOs is presented in Figure 4. It varies depending on the variety of olive tree, the agronomic conditions, e.g., soil and climate, the harvesting method, and the OO production system. OO consists of approximately 98% neutral lipids (NL), with the main component being triglycerides (TG), and 1–2% of polar lipids (PL) that contain a great variety of complicated chemical compounds. The terms saponifiable and nonsaponifiable fractions that are frequently used in oils generally are not identical in terms of composition with the fractions of NL and PL, respectively, since phytosterols, hydrocarbons, squalene, α-, β-, γ-tocopherols, and triterpenes that are present in the NL fraction are extracted in the nonsaponifiable fraction.
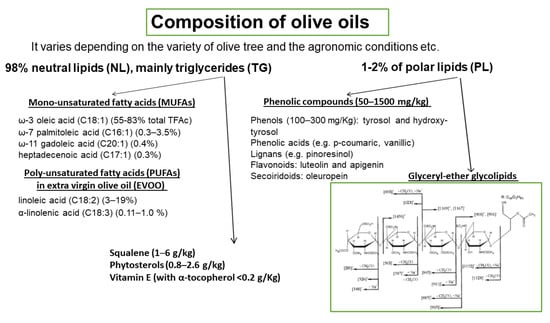
Among the microconstituents that belong to the class of NL, mono-unsaturated fatty acids (MUFAs), and especially oleic acid which is the main component of OO (60–83% of the total fatty acids), have attracted attention in relation to their beneficial effects. Other MUFAs are also present in smaller quantities (Figure 4). Extra virgin olive oil (EVOO) also contains significant amounts of poly-unsaturated fatty acids (PUFAs), with linoleic acid (3–19%) being the main one. Other microconstituents present in the NL are squalene (1–6 g/kg), phytosterols (0.8–2.6 g/kg), and vitamin E (with α-tocopherol as the predominant form, <0.2 g/Kg). The triterpenes, erythrodiol, uvaol, oleanolic, and maslinic acids, are found in very low concentrations in VOOs but are present in detectable amounts in olive pomace oil. PL fraction includes phenolic compounds (50–1500 mg/kg) that are classified into four main groups: (a) phenols (100–300 mg/Kg), tyrosol (T) and hydroxy-tyrosol (HT) and phenolic acids e.g., p-coumaric, vanillic; (b) lignans (1–100 mg/kg), e.g., pinoresinol; (c) flavonoids (luteolin and apigenin, 1–7.5 mg/kg); and (d) secoiridoids (4–5 mg/kg), oleuropein. Among the above compounds, HT is the most abundant one. The above amounts of phenolic compounds are present in VOO or EVOO. In a recent paper, a significant number of phospholipids are identified in EVOO, with phosphatidic acids and phosphatidylglycerols being the most abundant species [41]. Glyceryl-ether glycolipids are also included in the PL fraction and a representative structure is shown in Figure 4 [42]. In order to preserve the fraction of PL that includes phenolic compounds and glyceryl-ether glycolipids, oils must not be processed or refined.
2. Olive Oil By-Products
Olive pomace (OP) is the main by-product of the OO production process. It is a semi-solid residue, and it consists of pieces of skin, pulp, olive kernel, and some oil. Even though the type and the amount of OO by-product depends on the type of the extraction method, namely press method, two-phase, and three-phase system, it has serious environmental impact. The two-phase OO extraction process, which is characterized as ecological compared to the three-phase system, produces OP with a moisture content of 65%, also named “alperujo”, which accounts for approximately 80% of the olive weight.
OP contains carbohydrates mainly in the form of polysaccharides, lipids, phenolic compounds as well as inorganic compounds. It contains the same classes of phenolic compounds with the OO and the predominant ones are T, HT, and p-coumaric acid [118]. In addition, the fraction of OP PL includes PAF-inhibitors having the structure of glyceryl-ether glycolipids sharing similar biological activity and structurally characteristics to the ones from OO PL [119]. Besides its toxicity, OP is a source of valuable compounds, so a variety of methods—some of them as patents—have been developed in order to purify them and specially to obtain phenolic compounds. Their suggested applications range from nutritional supplements and functional foods to animal feed.
As mentioned above, the triterpenes, erythrodiol, uvaol, oleanolic, and maslinic acids, are present in detectable amounts in OP oil (OPO). These triterpenes are described mostly in in vitro studies but also in ApoE mice and rats, to present anti-inflammatory, antioxidant properties, vasodilatory properties mainly mediated by the endothelial production of NO as well as cardioprotective effects. The efficient doses range from 10 to 400 μM in the in vitro experiments while 50–100 mg/kg/day are supplemented in the feed of the experimental animals [120]. In a randomized, crossover trial, healthy adults consumed for three weeks, a daily dose (30 mL) of three OOs, namely a standard VOO (124 ppm of phenolic compounds and 86 ppm of triterpenes) as a control, a VOO fortified with phenolic compounds (490 ppm of phenolic compounds and 86 ppm of triterpenes), and another VOO fortified with both phenolic compounds and triterpenes (487 ppm of phenolic compounds and 389 ppm of triterpenes). The results demonstrated that the VOO with the highest triterpenes amount had no additionally effect in biomarkers related to metabolic syndrome and endothelial function [121], while its consumption resulted in decreased DNA oxidation and plasma IL-8 and TNFα concentrations compared to the other two oils [122]. These results are also confirmed by a randomized, blind, crossover, controlled, clinical trial, in healthy and hypercholesterolemic participants that consumed daily 45 g of either OPO or high-oleic acid sunflower (HOSO) oil for 4 weeks. Both oils had comparable oleic acid content while tocopherols and sterols were higher in HOSO and OPO and showed a higher content of aliphatic alcohols, squalene, and triterpenes. The intake of OPO resulted in lowered LDL-cholesterol and Apo B levels compared to the HOSO but without affecting blood pressure, endothelial function as well as a significant number of inflammatory biomarkers [123].
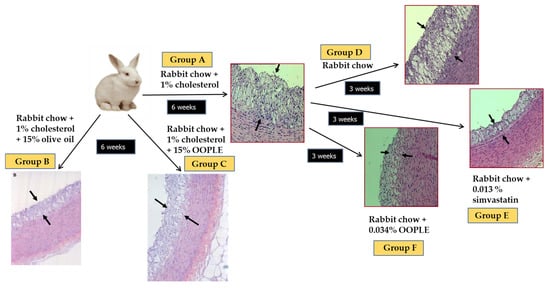
Our research group has demonstrated that the PL fraction from the olive pomace (OO polar lipid extract, OOPLE) inhibited in vitro PAF-induced platelet aggregation with an IC50 value equal to 1.1 × 10−10 M, expressed as sugars, and equivalent to the corresponding one induced by the PL from OO (IC50 PAF 1.5 × 10−10 M, expressed as sugars). This inhibition is attributed to binding to the PAF-R with EC50 value at 0.42 × 10−7 M (expressed as sugars) which is comparable to the one of the well-known PAF-R inhibitors, BN 52021 (EC50 = 2.3 × 10−7 M), a ginkgolide of the Chinese plant Gingko biloba. In addition, the OOPLE had the ability not only to inhibit the formation of atheromatous plaques in hypercholesterolemic rabbits, but also to improve artery elasticity and to reduce the thickness of the already formed atheromatous plaques comparable to simvastatin, which was used as a positive control (Figure 5) [124]. In order to investigate the effect of the OOPLE in humans, a low-fat yogurt was enriched by 0.4%, w/w with this fraction. The randomized, three-arm, double-blind, placebo-controlled, parallel-group trial, performed in apparently healthy and mainly overweight adults, showed that the daily intake of the yogurt supplemented with OOPLE (150 g) for a period of eight weeks resulted in lower levels of inflammatory cytokines and in reduced platelet sensitivity against PAF, compared to plain yogurt, while no impact on energy intake, body weight, glucose, and lipid metabolism was detected [125]. Additionally, the intake of the enriched yogurt favourably modulated PAF metabolism by reducing PAF-CPT and LpPLA2 activities [126]. In addition, diet supplementation with exactly the same enriched yogurt (2%, w/w) in rabbits fed with a cholesterol-enriched diet resulted in a decrease in the maximum and average thickness of atheromatous plaques by 54% and 57%, respectively [127]. Finally, preliminary results from another intervention study showed that the consumption for 4 weeks of fillets from fish fed with a diet, where fish oil/meal in the grow-out feed of gilthead sea bream was partly replaced with the OOPLE, led to reduction of platelet sensitivity against PAF, and ADP by 44% and 67%, respectively, compared to consumption of fillets from fish fed the typical diet [128]. The reduced platelet sensitivity observed in both trials after the intake of the OOPLE rich in PAF antagonists, is in accordance with the results from our previous intervention studies where the same effect was recorded in healthy subjects and type II diabetic patients after one-month consumption of Mediterranean-type foods rich in PAF inhibitors [129,130]. The above experiments with OOPLE highlight the value of using OOPLE as a food supplement and/or ingredient to add to create functional foods. In addition, there is also an environmental interest since a high added value component (OOPLE) is isolated from industrial waste from olive oil production.
Apart from OP, two human trials have been performed with the olive mill wastewater (OMWW). In the first one that was a non-placebo postprandial intervention, healthy subjects consumed 2 mL of a commercially available OMWW preparation containing approximately 50 mg of HT and 8 mg of oleuropein along with other phenolic compounds. The results showed that the glutathione plasma concentration increased, while no difference in plasma antioxidant capacity was detected [131]. In the second study, five type I diabetic patients received 25 mg of HT the first day and 12.5 mg/day the following three days as a HT-rich phenolic extract from OMWW along with breakfast. The results demonstrated a significant decrease in the serum TXB2 production after blood clotting at the end of the intervention [132].
In conclusion, VOO shows anti-atherogenic effects, which can be attributed to the synergistic action of its microconstituents, mainly PL that act as PAF inhibitors, tyrosol and oleuropein as well as T that also exert anti-PAF activity. Finally, as the EFSA suggests the daily intake of moderate amounts of OO that can be easily consumed in the context of a balanced diet is considered satisfactory for the health of the general population [133].
This entry is adapted from the peer-reviewed paper 10.3390/biom13040700
