Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
水凝胶敷料领域最先进、最有前途和商业上可行的研究问题之一是获得功能,以实现改善的治疗效果甚至智能伤口修复。功能性水凝胶敷料除了具有普通水凝胶敷料的优点外,还可以调整其化学/物理性质以满足不同的伤口类型,进行相应的反应,积极创造有利于伤口修复的愈合环境,还可以控制药物释放以提供持久的益处。
- hydrogel dressing
- functionality
- wound repair
1. Dynamic cross-linked hydrogel dressing
Traditional dressings lack the advantages of self-healing and injectability in clinical practic[33].由Due to the rigidity and non dynamic nature of the skeleton, the hydrogel is easy to deform or damage due to external forces, which shortens its service life and makes it difficult to maintain full contact with the wound, especially near the joint, resulting in unsatisfactory treatment effect[34,35].For example, traditional dressings attached to fingers cannot fit well with finger wounds and are prone to deformation and detachment due to frequent bending of the fingers[36].Injectable hydrogels can fill irregular wound areas and promote in situ tissue regeneration. Self repairing hydrogels can withstand external mechanical forces, thus extending their service life[37].Therefore, in order to promote a favorable environment for wound repair, functional hydrogel dressings should have a higher ability to resist mechanical injury and debridement[38](Figure 3a).
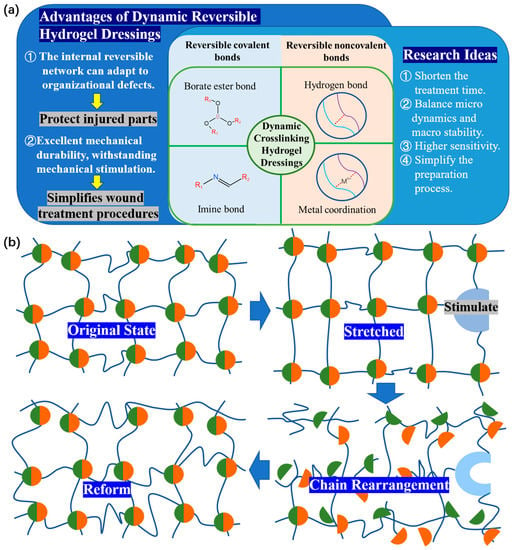
Figure 3.(a)The research idea and(b) principle of self-healing and injectability of dynamic reversible gel dressing.
Hydrogels with self-healing and injectable properties can be prepared together through non covalent cross-linking or dynamic covalent cross-linking[39](Figure 3b).根据动态可逆水凝胶敷料的研发成果,张团队为动态水凝胶的进一步发展提出了一些研究思路[40]:(<>)需要减少动态应用伤口治疗所需的时间。(<>)要在微观动态和宏观稳定之间取得更好的平衡。(<>)需要获得更高的灵敏度来实时表征水凝胶的动态特性。(<>)要简化水凝胶制备工艺,促进动态水凝胶在基础研究和临床转化中的广泛应用。这些想法为进一步开发功能性动态可逆凝胶敷料和伤口修复指明了方向。
在讨论动态水凝胶敷料性能的一般期望和设计原则时,应考虑水凝胶敷料的宏观稳定性和微观水平动力学与敷料应用要求之间的平衡。在实践中,最常见的可逆共价键是亚胺键。还有硼酸酯键,特殊的动态共价键,在室温下可以在几秒钟内发生键交换反应[41]。金属配位键和氢键是可逆非共价键的两个最典型的例子。这将作为本节的起点,介绍该领域当前研究的成功。
2. 可逆共价键
可逆共价键通常是可逆的,这意味着可以发生键交换。根据大量研究,水凝胶敷料可以通过动态可逆共价键恢复[42],证明了其自我修复和注射性的潜力。
2.1. 亚胺键
自1864年希夫首次成功制备亚胺键以来,研究人员对亚胺键进行了深入研究,积累了大量的研究经验[43]。亚胺键具有动态可逆性质。这些官能团之间的动态可逆共价键是水凝胶自愈的主要驱动力,而不是断裂的水凝胶界面之间的简单物理粘附[44]。通过亚胺键动态交联的水凝胶敷料具有出色的注射性和高效的自愈能力[45]。
在早期的研究中,Chen等[46]使用多巴胺接枝氧化海藻酸钠(OSA-DA),聚丙烯酰胺(PAM)和盐酸多巴胺(DA)作为主要原料制备OSA-DA-PAM水凝胶敷料。通过动态共价交联OSA-DA链的醛基和PAM链的氨基之间形成的碱反应来合成水凝胶敷料(图4a)。由此产生的水凝胶可以在没有任何外部刺激的情况下有效地自我修复。然而,这项研究的主要弱点是未能解决注射性问题。需要找到在自愈和注射性方面都出色的其他制备方法。
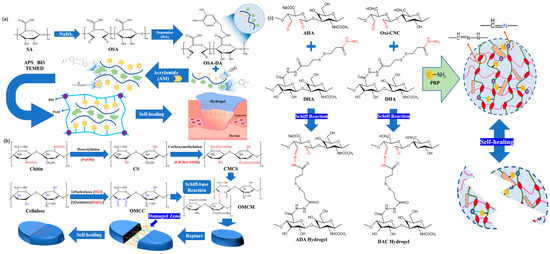
图4.(a) OSA-DA-PAM水凝胶的合成工艺和结构图。(b) 基于希夫碱反应和自愈过程方案制备OMCM水凝胶。(c) AHA/DHA/氧数控水凝胶示意图。
鉴于先前研究中遇到的问题,Yin 等人 [47] 发表了一篇论文,其中描述了使用来自菠萝皮 (PP) 的氧化微晶纤维素 (OMCC) 的醛基与来自猴头菇残基 (HER) 的羧甲基壳聚糖 (CMCS) 的氨基交联构建的水凝胶敷料,并使用希夫碱反应构建(图 4由于亚胺键的不断破裂和再生,研究人员将水凝胶敷料组成的网络分为两部分,需要5 h才能实现自我修复。对于伤口敷料的要求,本品的自愈时间太长。动态共价交联可以在水凝胶敷料中通过使用促进可逆共价键形成和交换的特定产品或组分来实现。这些动态共价交联相互作用有助于水凝胶敷料的自愈和可注射特性。另一方面,共价交联的水凝胶敷料在注射过程中不被堵塞,显示出良好的注射性能。这两个特征有助于减轻疼痛并加速患者的愈合过程[48]。同样,通过使用亚胺键,Li等人[49]产生了由醛改性透明质酸钠(AHA),肼改性透明质酸钠(ADA)和醛改性纤维素纳米晶体(oxi-CNC)组成的自修复水凝胶敷料(图4c)。当这些水凝胶敷料被分成两块时,它们可以自我修复并在大约4小时后重新连接成一个有凝聚力的碎片。然而,这两个项目的自我修复时间仍然不是最佳的。
为了提高水凝胶敷料的注射能力和自愈能力,Ding等[50]证明胶原蛋白(CoL)-壳聚糖(CS)复合水凝胶可应用于医疗领域的多功能伤口敷料。在他们的工作中,二苯甲醛改性PEG2000(DA-PEG)是一种长距离交联剂,被引入混合的COL-CS网络中,并用作动态交联剂(图5a)。这些特性有助于动态解耦和重新耦合亚胺连接,从而产生卓越的自修复水凝胶敷料。借助这种制备技术,功能性水凝胶敷料的自愈时间可以显着减少,同时仍保持良好的注射性。这是在水凝胶敷料中应用亚胺键的关键阶段。
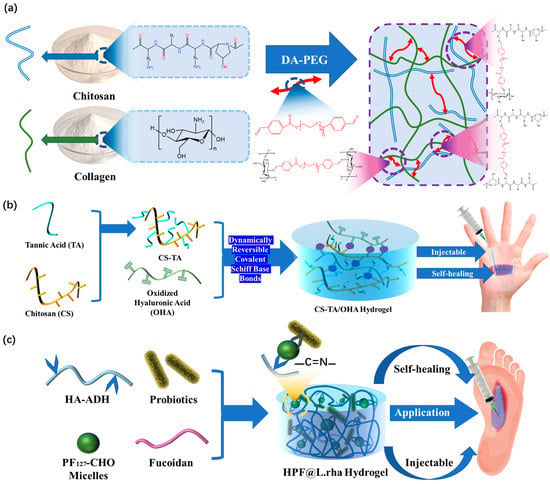
Figure 5. (a) Construction mechanism of COL-CS hydrogel. (b) The construction mechanism and functional mechanism of injectable and self-healing HPF@L.rha hydrogel dressings. (c) Design strategy and application method of CS-TA/OHA composite hydrogel dressings.
Previous research on the injectability and self-healing ability of hydrogels did not address the restoration of the original hydrogel’s mechanical properties. Exceptional performance of both properties is not only necessary to integrate the hydrogel dressing into a whole, but also to restore its excellent mechanical properties, such as its good tensile properties [51]. In order to achieve leapfrogging improvements, Mei et al. [52] modified hyaluronic acid (HA) with adipic hydrazide through acetylation to synthesize hyaluronic acid adipic hydrazide (HA-ADH). (Pluronic F127)-CHO and HA-ADH form dynamic covalent cross-links through Schiff base reaction interactions in the interventricular or physiological conditions (Figure 5b). The formation of the dynamic imine connection is what gives the dressing its self-healing property. It can bear high stress and can easily reform at low strain levels; meanwhile, the self-healing time is significantly reduced. In addition, other studies have further improved the performance of hydrogel dressings constructed by imine bonds. Using the dynamic reversible characteristics of Schiff base bonds between chitosan (CS), tannic acid (TA), and oxidized hyaluronic acid (OHA) [53], Liu’s hydrogel dressings were able to achieve traceless repair in a rather short time, which has a very short self-healing time compared to recent reports (Figure 5c). This excellent treatment time is a noteworthy achievement for dynamic reversible gel dressings prepared by imine bonds. It quickly achieves untraceable repair and has good injectability.
2.2. Boric Acid Ester Bonds
Boric acid ester bonds are a special type of dynamic covalent bond that may change dynamically and reversibly at room temperature [54]. The bond exchange and repair times are also substantially shorter than those for imine bonds [55], which enhances their capacity for self-healing.
This hydrogel material is based on a dynamically reversible borate ester bond, and borax is used as a catalyst and dynamic crosslinking agent, expanding the design, formulation, and development possibilities and making it suitable for new hydrogel systems [56]. This prepared hydrogel is favorable for applications in wound dressings. More importantly, it provides a novel idea that is superior to the imine bond. The excellent self-healing ability of the borate ester bond has been demonstrated in the field of wound repair.
In order to demonstrate the advantages of borate bonds in the preparation of self-healing functional hydrogel dressing, Zhong et al. [57] used dopamine-grafted oxidized carboxymethyl cellulose (OCMC-DA) and cellulose nanofibers (CNF) to build a dynamic reversible borate ester bond to produce a hydrogel dressing that could quickly complete self-healing in 12 s and had certain injectability (Figure 6a). This type of excellent hydrogel dressing also accomplishes the function of degradation. The same research group has other achievements in the research of hydrogel dressings [58]. Through the dynamic covalent bond between boric acid and a catechol group, the quaternized chitosan can be loaded with epigallocatechin-3-gallate (EGCG), which can be used to meet the requirement of rapid self-healing (Figure 6b). Within 5 min, a hydrogel dressing that has been cut can be automatically reintegrated into one piece. If the injury is just a scratch, the scratch will vanish entirely from the healing interface after 20 s, realizing complete recovery. The adaptability and durability of this hydrogel as a wound dressing are significantly enhanced by its strong self-healing capacity. Compared to the aforementioned hydrogel dressings, its injectable properties are superior. Moreover, this hydrogel dressing also has antioxidant properties and a powerful bactericidal effect.
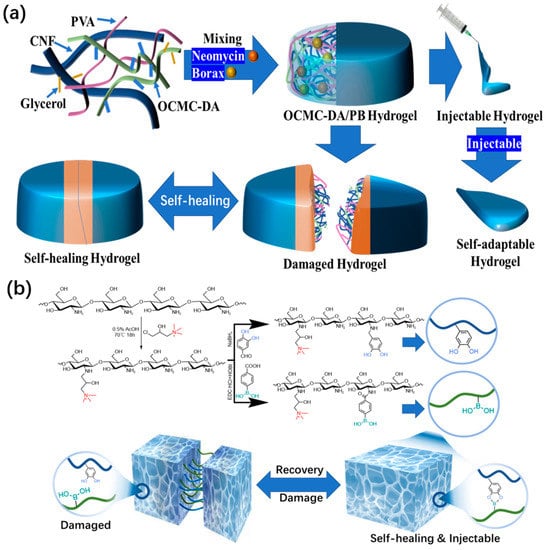
Figure 6. (a) Schematic illustration and microscopic structure of OCMC-DA/PB hydrogel and an application diagram of a self-healing and injectable hydrogel. (b) Schematic diagram of QCS-PC hydrogel preparation and the application of self-healing and injectable properties.
Other research groups have studied this type of hydrogel dressings. Deng et al. [59] found that hydroxypropyl cellulose and phenylboronic acid-modified hydrogels could be constructed through dynamic borate bonds (Figure 7a). The prepared hydrogel dressing has excellent self-healing performance. The PAHC (phenylboric acid-modified hydroxypropyl cellulose) hydrogel completely healed after 10 min, which proves that it has excellent self-healing ability. Through continuous research, the research team has made preliminary achievements in the research and development of self-healing and injectable dynamic reversible gel dressings. However, the self-healing time of these dressings still needs to be improved. In 2022, Deng’s group [60] grafted 4-carboxylphenylboronic acid onto the molecular chain of hydroxypropyl chitosan (HPC) through amidation. The dynamic borate ester bonds are formed between phenylboronic acid and the catechol structure of polydopamine (PDA)-modified carbon nanotubes (Figure 7b). The final product has excellent self-healing ability compared to the other self-healing hydrogel dressing products mentioned above. The new hydrogel dressing developed by Deng’s group in 2022 can heal itself immediately. The new product developed by this team is more suitable for wound repair than other self-healing hydrogel dressings mentioned previously. This is because the self-healing time of the dressing has been significantly reduced compared to its previous iterations.
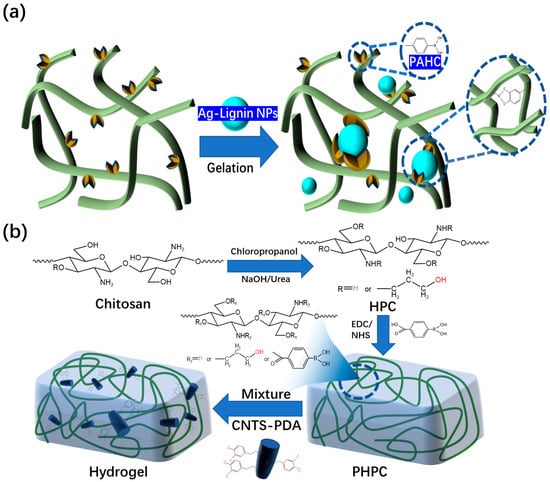
Figure 7. (a) Microstructure of hydrogels composed of PAHC and lignin-reduced Ag NPs. (b) Preparation mechanism of PHPC-CNT hydrogel dressing and its microscopic model.
3. Reversible Noncovalent Bonds
Dynamic reversible hydrogel dressings can also be composed of reversible noncovalent bonds, such as metal coordination bonds and hydrogen bonds. These interactions are usually always in the dynamic state of fracture and reorganization, which can achieve rapid and repeated recovery [61].
3.1. Metal Coordination Bond
Hydrogels with self-healing properties were prepared by forming cross-linking points based on ligand–metal coordination bonds on the polymer skeleton. By choosing different ligands and metal ions, a variety of hydrogels with different mechanical strengths can be made to satisfy the self-healing requirements of various biomedical applications [62].
Copper ions are a common material used for metal coordination crosslinking in reams of research. Using hydrazide group as ligand and Cu2+ as coordination center, Qian et al. [63] developed a copper–hydrazide-coordinated, multifunctional hyaluronan hydrogel, and the hyaluronic acid (HA)-Cu hydrogel shows practical versatility, including excellent self-healing and injectability (Figure 8a). Self-healing is achieved after 0.5 h, and the self-healing hydrogel can even be stretched during this time. From the perspective of hydrogel restoration, the HA-Cu hydrogel also has good injectability. Most importantly, this product is not restricted to only reacting in acidic conditions. This is the first report on hydrogels prepared by aliphatic hydrazine–metal coordination crosslinking for wound treatment.
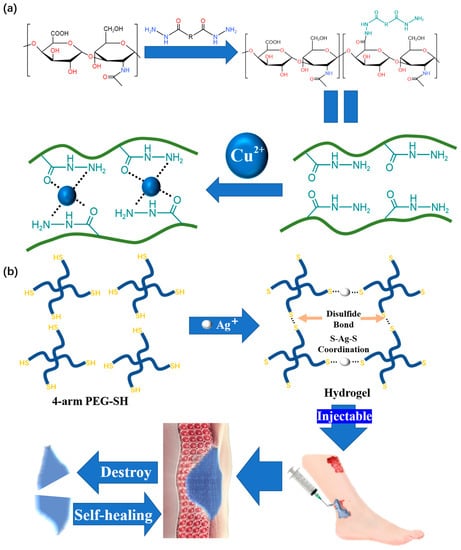
Figure 8. (a) The fabrication of HA hydrogels through hydrazide–metal coordination crosslinking and its microstructure. (b) Schematic illustration of the self-healing Ag(I)-thiol (Au–S) coordinative hydrogel and its self-healing and injectable applications.
We should also note that silver is frequently used for metal coordination crosslinking. Compared with Cu, Ag is more suitable for wound treatment due to its wider antibacterial spectrum and stronger antibacterial ability [64]. The hydrogel developed by Chen [65], which can be used for wound treatment, is prepared by the coordination crosslinking of dobby thiopenyl glycol (SH-PEG) and silver nitrate (AgNO3) (Figure 8b). Due to the dynamic nature of the Ag-S coordination bond, the obtained coordination hydrogels have good self-healing ability and injectability. At room temperature, the hydrogel shows excellent self-healing performance by restoring its original state from an incision within 15 min. A unique feature of the prepared hydrogel is its ability to have its mechanical strength adjusted, which is useful for adapting to various wound conditions.
Fe3+ is very common, and the raw materials are cheap. In addition, Fe3+ can also be converted into Fe2+ in an acidic environment [66] (human skin is generally weakly acidic), which is helpful for the blood supply in the human body during wound healing. Moreover, this crosslinking method is environmentally friendly, simple, and universal. Based on the application of metal coordination bonds to prepare hydrogel dressings, the double dynamic bond crosslinked self-healing hydrogel prepared by Liang’s team [67] used the tricomplex coordination between the catechol groups from Fe3+ and protocatechualdehyde (PA) to produce hydrogels with excellent self-healing performance and injectability (Figure 9a). The resulting hydrogel dressing is intelligent and the wound repair efficiency is improved. The performance test revealed that the hydrogel could be fully repaired after 30 min of room temperature exposure and that its tensile capacity could be restored after an additional hour. It was proven that the reversible non-covalent cross-linked hydrogel dressing prepared by metal coordination has a certain self-healing ability, but the healing efficiency still needs to be improved.
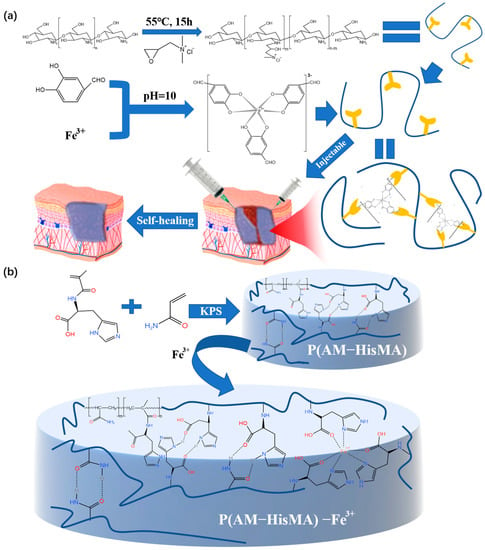
Figure 9. (a) The process of preparing double-dynamic-bond crosslinked hydrogels and the application of the product in medicine. (b) The preparation and the network structure of the P(AM-HisMA) −Fe3+ hydrogel dressing.
Wound repair requires fast self-healing of the dressing in order to better repair the wound. Therefore, after using histidine methacrylamide (HisMA) and acrylamide (AM) to form hydrogel, Zhang [68] added Fe3+ ions into a P(AM-HisMA) hydrogel to prepare a P(AM-HisMA)-Fe3+ hydrogel dressing with good injectability and self-healing ability (Figure 9b). It is worth noting that the self-healing speed is greatly improved compared to the hydrogel without Fe3+. P(AM-HisMA)-Fe3+ hydrogel completely heals in 5 min; however, the P(AM-HisMA) hydrogels without Fe3+ crosslinking take 24 h to achieve self-healing. Since its capacity for self-healing is an important reference scheme for this kind of hydrogel dressing, it merits special attention.
3.2. Hydrogen Bond
Another type of dynamic noncovalent bond, the hydrogen bond [69], is a noncovalent bond that can be destroyed at high temperature and reformed at low temperature. Therefore, hydrogen bonds can be used to prepare self-healing hydrogel dressings.
In order to improve the self-healing ability and injectability of functional hydrogel dressings, Zhao [70] developed a simple and fast method to prepare a new hydrogel. This hydrogel is composed of sodium alginate (SA) and polyacrylamide (PAM). Systematic characterization revealed the formation mechanism of a layered structure through hydrogen bonding (Figure 10a). In addition, it takes 0.5 h to achieve self-healing. Hydrogen bonds allow for the recovery of the mechanical properties of hydrogels with or without the presence of water.
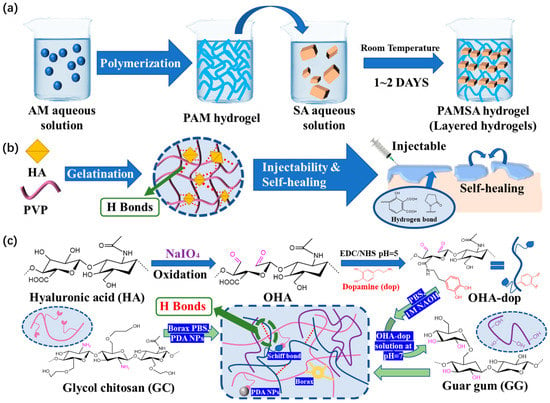
Figure 10. (a) Schematic diagram of PAMSA hydrogel preparation based on PAM and SA. (b) Schematic diagram of HPC hydrogel crosslinked by HA in an ultrafast process and the possible hydrogen bonding structure of HPC hydrogels. (c) The preparation and microscopic schematic diagrams of the OHAdop/GG + GC/borax hydrogel and the OHAdop/GG + GC/borax/PDA hydrogel.
There are also better research results on the self-healing ability and injectability of hydrogel dressings. Among dynamic crosslinking hydrogel dressings with these properties, the humic acid/polyvinylpyrrolidone (PVP) complex hydrogel dressings prepared by Yu et al. [71] are driven by the hydrogen bonds between humic acid and PVP, and these bonds are easy to form and adjust (Figure 10b). The dynamic reversible hydrogen bonds distributed in the hydrogel dressing promoted the formation of a dynamic cross-linking network. The viscosity of the humic acid/PVP complex hydrogel significantly decreases as the shear rate rises, improving its injectability. At the same time, this hydrogel is endowed with self-healing performance that can achieve complete self-repair in 10 min.
Because the force of hydrogen bonds is generally weaker than some of the dynamic reversible bonds discussed above, hydrogen bonds often do not appear alone; rather, hydrogels are constructed combing hydrogen bonds with other dynamic cross-linking bonds. Guo et al. [72] designed and prepared a polysaccharide-based adhesive hydrogel, in which dynamic hydrogen bonding between catechol-modified oxidized hyaluronic acid (OHAdop) or hydroxide groups in guar gum led to reversible crosslinking (Figure 10c). As soon as the two parts come into contact, hydrogen interaction occurs, causing the fragments to self-heal and unite to form a single hydrogel that can be easily combined with other parts. This hydrogel dressing heals itself extremely quickly. It has high application value and competitiveness in the market.
This entry is adapted from the peer-reviewed paper 10.3390/polym15092000
This entry is offline, you can click here to edit this entry!
