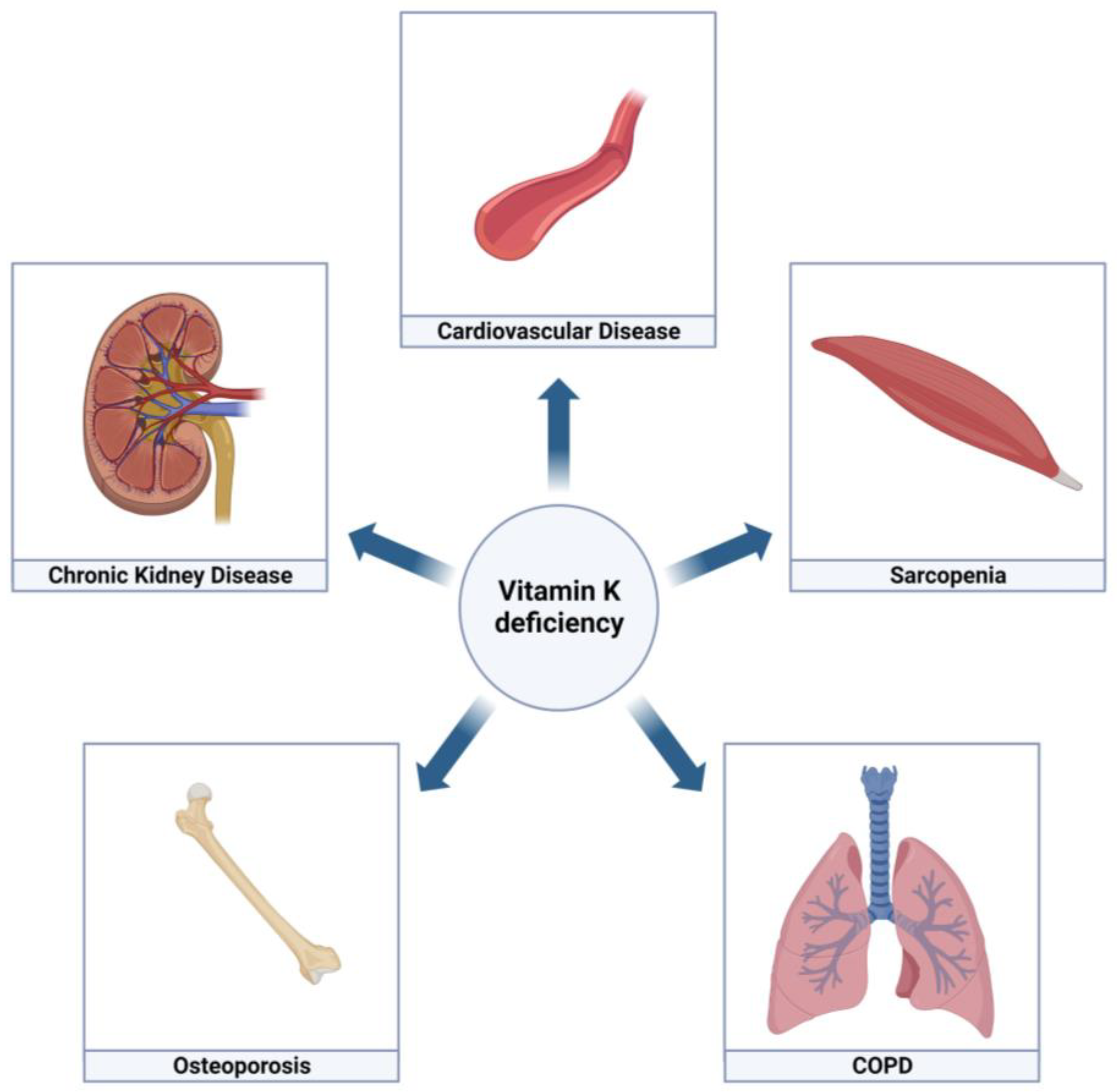
Although defined by the presence of airflow obstruction and respiratory symptoms, patients with chronic obstructive pulmonary disease (COPD) are characterized by multimorbidity. Numerous co-occurring conditions and systemic manifestations contribute to the clinical presentation and progression of COPD. Vitamin A and vitamin D have been related to COPD pathogenesis. Another fat-soluble vitamin, vitamin K, has been put forward to exert protective roles in COPD. Vitamin K is an unequivocal cofactor for the carboxylation of coagulation factors, but also for extra-hepatic proteins including the soft tissue calcification inhibitor matrix Gla-protein and the bone protein osteocalcin.
- chronic obstructive pulmonary disease
- emphysema
- vitamin K
1. Introduction
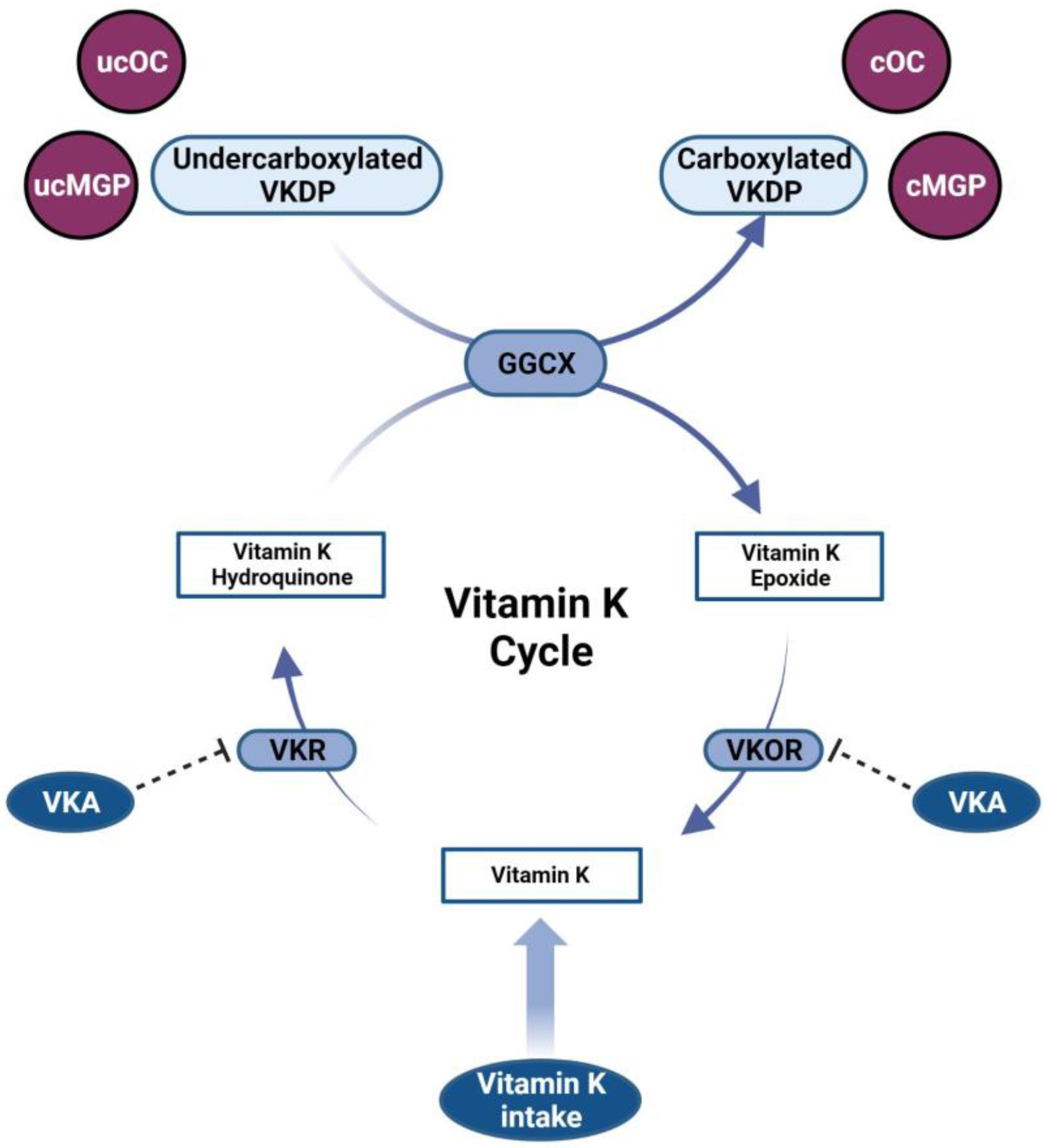
2. Vitamin K
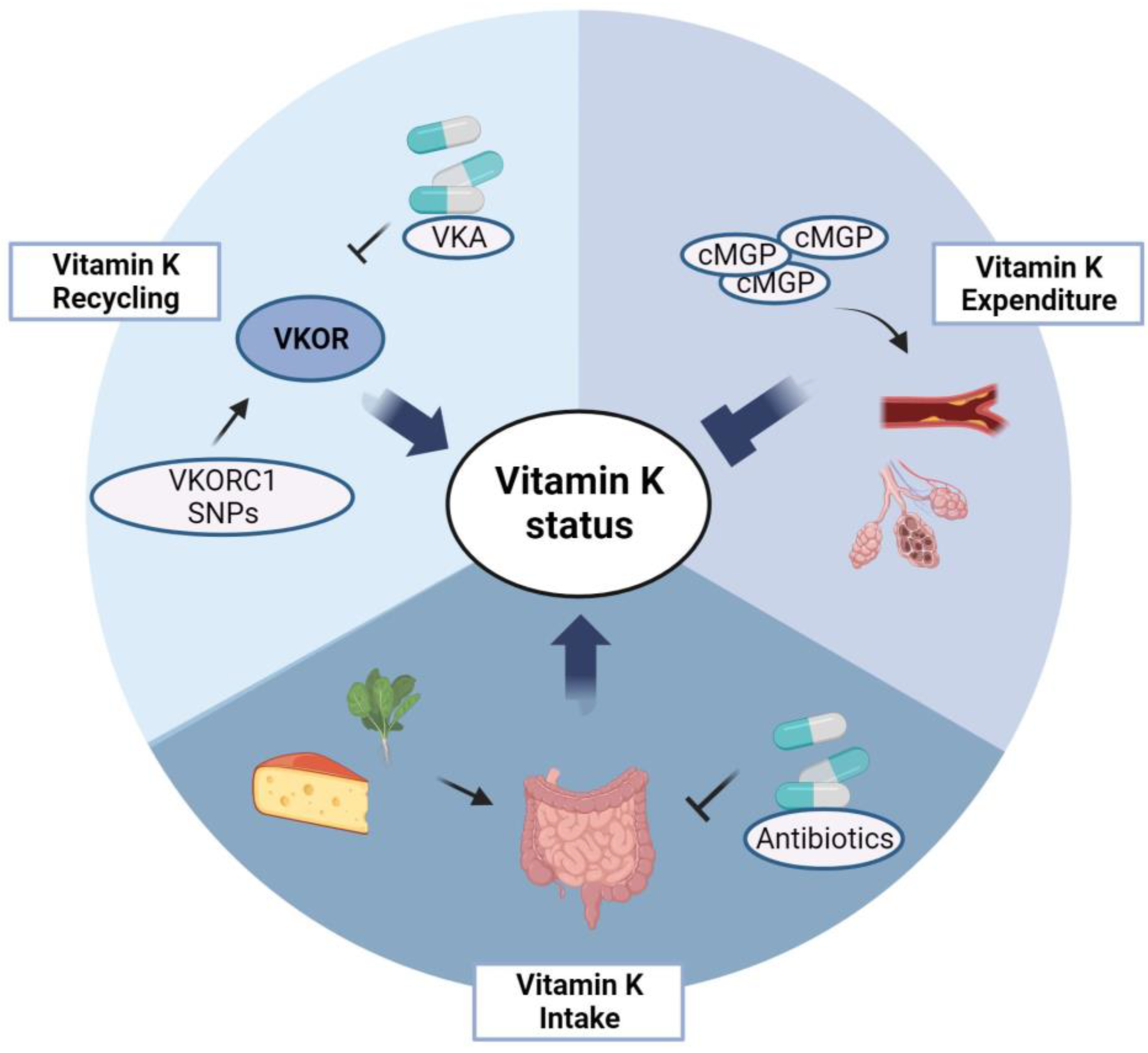
3. Vitamin K in COPD
This entry is adapted from the peer-reviewed paper 10.3390/jcm12041261
References
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582.
- Vanfleteren, L.E.; Spruit, M.A.; Groenen, M.; Gaffron, S.; van Empel, V.P.; Bruijnzeel, P.L.; Rutten, E.P.; Op’t Roodt, J.; Wouters, E.F.; Franssen, F.M. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 728–735.
- Triest, F.J.J.; Franssen, F.M.E.; Reynaert, N.; Gaffron, S.; Spruit, M.A.; Janssen, D.J.A.; Rutten, E.P.A.; Wouters, E.F.M.; Vanfleteren, L.E.G.W. Disease-Specific Comorbidity Clusters in COPD and Accelerated Aging. J. Clin. Med. 2019, 8, 511.
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. 2013, 22, 454–475.
- Bernardo, I.; Bozinovski, S.; Vlahos, R. Targeting oxidant-dependent mechanisms for the treatment of COPD and its comorbidities. Pharmacol. Ther. 2015, 155, 60–79.
- Negewo, N.A.; Gibson, P.G.; McDonald, V.M. COPD and its comorbidities: Impact, measurement and mechanisms. Respirology 2015, 20, 1160–1171.
- Macnee, W.; Maclay, J.; McAllister, D. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2008, 5, 824–833.
- Caram, L.M.; Amaral, R.A.; Ferrari, R.; Tanni, S.E.; Correa, C.R.; Paiva, S.A.; Godoy, I. Serum Vitamin A and Inflammatory Markers in Individuals with and without Chronic Obstructive Pulmonary Disease. Mediators Inflamm. 2015, 2015, 862086.
- Jolliffe, D.A.; Greenberg, L.; Hooper, R.L.; Mathyssen, C.; Rafiq, R.; de Jongh, R.T.; Camargo, C.A.; Griffiths, C.J.; Janssens, W.; Martineau, A.R. Vitamin D to prevent exacerbations of COPD: Systematic review and meta-analysis of individual participant data from randomised controlled trials. Thorax 2019, 74, 337–345.
- Fairfield, K.M.; Fletcher, R.H. Vitamins for chronic disease prevention in adults: Scientific review. JAMA 2002, 287, 3116–3126, Erratum in JAMA 2002, 288, 1720.
- Stafford, D.W. The vitamin K cycle. J. Thromb. Haemost. 2005, 3, 1873–1878.
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226.
- Nagata, C.; Wada, K.; Tamura, T.; Konishi, K.; Goto, Y.; Koda, S.; Kawachi, T.; Tsuji, M.; Nakamura, K. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: The Takayama study. Am. J. Clin. Nutr. 2017, 105, 426–431.
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32.
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. The role of menaquinones (vitamin K₂) in human health. Br. J. Nutr. 2013, 110, 1357–1368.
- Vermeer, C. Vitamin, K: The effect on health beyond coagulation—An overview. Food Nutr. Res. 2012, 56, 5329.
- Crosier, M.D.; Peter, I.; Booth, S.L.; Bennett, G.; Dawson-Hughes, B.; Ordovas, J.M. Association of sequence variations in vitamin K epoxide reductase and gamma-glutamyl carboxylase genes with biochemical measures of vitamin K status. J. Nutr. Sci. Vitaminol. 2009, 55, 112–119.
- Tie, J.K.; Stafford, D.W. Structure and function of vitamin K epoxide reductase. Vitam. Horm. 2008, 78, 103–130.
- Shea, M.K.; Booth, S.L. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients 2016, 8, 8.
- McCann, J.C.; Ames, B.N. Vitamin K an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907.
- Shen, T.; Bimali, M.; Faramawi, M.; Orloff, M.S. Consumption of Vitamin K and Vitamin A Are Associated With Reduced Risk of Developing Emphysema: NHANES 2007–2016. Front. Nutr. 2020, 7, 47.
- Piscaer, I.; van den Ouweland, J.M.W.; Vermeersch, K.; Reynaert, N.L.; Franssen, F.M.E.; Keene, S.; Wouters, E.F.M.; Janssens, W.; Vermeer, C.; Janssen, R. Low Vitamin K Status Is Associated with Increased Elastin Degradation in Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2019, 8, 1116.
- Piscaer, I.; Kalkman, G.A.; Fleuren, H.W.; Janssens, W.; Wouters, E.F.; Franssen, F.M.; Janssen, R. Use of Vitamin K Antagonists Is Associated with Increased Mortality in Chronic Obstructive Pulmonary Disease . Am. J. Respir. Crit. Care Med. 2018, 197, A4234.
