Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chemical modification and self-assembly of molecules as well as constructing porphyrin-based metal (covalent) organic frameworks are often used to improve its solar light utilization and electron transfer rate. Especially porphyrin-based covalent organic frameworks (COFs) in which porphyrin molecules are connected by covalent bonds combine the structural advantages of organic frameworks with light-capturing properties of porphyrins and exhibit great potential in light-responsive materials.
- bio-inspired
- porphyrin-based COFs
- photoelectric conversion mechanism
1. Introduction
Overreliance on fossil fuels leads to growing energy crises and environmental problems. It is imperative to develop clean and renewable energy, such as solar energy, instead of traditional fossil fuels. Organisms, including green plants and certain microorganisms, show great skill in solar energy utilization, in which solar energy is converted to chemical energy and stored in carbohydrates. Inspired by natural photosynthesis, photocatalytic conversion for solar water splitting and CO2 reduction to produce hydrocarbon fuels has been considered one of the most promising ways to solve the increasingly serious energy and environmental problems [1][2][3][4]. Since photocatalyst plays a pivotal role in the photocatalysis reaction, the design and preparation of highly efficient photocatalysts are important to improve solar energy utilization and photoelectric or photochemical conversion efficiency. An excellent photocatalyst requires not only light response in a wider wavelength range and high light absorption coefficient but also high photoelectric conversion and separation/transfer efficiency of charge carriers in the same substance and interface, appropriate electronic energy level, and redox gradient [5][6].
Since Fujishima and Honda first reported the photocatalytic decomposition of water on TiO2 electrodes in 1972 [5], many types of photocatalysts, including inorganic and organic photocatalysts, have been explored [7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]. Inorganic semiconductors represented by metal oxides such as TiO2 have the virtues of high stability and low price. However, certain inherent defects, such as wide band gap and mismatch of conduction/valence band position, result in poor visible light utilization and low quantum yield, which limit their photocatalytic performance [16][24][25][26][27]. Different from inorganic semiconductors, organic photocatalysts have abundant structural units and flexible designability. For example, plants show excellent solar light utilization dependent on the light-capturing ability of chlorophyll. As is known, chlorophyll is a derivative of porphyrin, and its large conjugated heterocyclic structure endows it with excellent photosensitivity. At the same time, nitrogen atoms in the porphyrin ring can coordinate with different metals. Such unique structures give them high versatility and adjustability [28][29]. Inspired by natural photosynthesis, it is expected to obtain stable and efficient photocatalysts by introducing porphyrin compounds into the photocatalytic system [30][31].
However, the intrinsic gap between the B and Q bands of porphyrin monomers locates in the strongest output range of the sun’s total irradiance spectrum (450–550 nm), which limits their light-capturing ability. Meanwhile, low charge carrier separation and transmission efficiency limit the development of porphyrin-based photocatalytic systems [32][33]. Chemical modification or combination with other dyes can improve their absorption in the visible light region. As is known, aggregation of porphyrin molecules can change their light absorption behavior. H-aggregation (face-to-face) leads to the blue shift, while J-aggregation results in the red shift. Therefore, an alternative method for changing the spectral properties is to regulate their aggregation state. J-aggregation of porphyrin leads to the red shift of the absorption maximum to the higher energy region of sunlight. Moreover, the J-aggregation of porphyrin allows the photogenerated electrons to spread over the J-aggregates [34][35][36][37][38][39][40][41][42]. Many efforts had devoted to constructing porphyrin J-aggregates, and we know that their photocatalytic ability can be improved by introducing structural regulatory reagents [31][43][44][45][46][47], replacing or modifying peripheral groups [48][49][50][51], complexing [52][53], choosing solvents appropriately [54][55], or changing the properties of solution [56][57][58][59]. Unfortunately, the J-aggregates of porphyrin are vulnerable to environmental conditions such as pH and ionic strength because most J-aggregates are stabilized by intermolecular electrostatic interactions. Additionally, defects in the aggregates limit the delocalization of photoinduced electrons and result in lower transfer efficiency. Therefore, more efforts are needed to construct stable and efficient porphyrin-based photocatalytic systems.
Compared to the porphyrin J-aggregates stabilized by electrostatic interactions, porphyrin-based polymers connected by covalent bonds are expected to be more stable. Especially for those connected with conjugated bonds, the conjugate structure allows the photoinduced electrons to delocalize along the polymer chain, which improves the transport rate of carriers. Porphyrin-based covalent organic frameworks (COFs) possess highly conjugated structures and π-π packing and unique performances in electron transport by regulating the porphyrin units at the molecular scale [60][61]. At the same time, the material has functional designability and structural controllability, easy to adjust and optimize the band structure and surface property of porphyrin-based COFs at the molecular level, thus enabling efficient light absorption and charge separation [62][63][64][65]. Corresponding to their ordered structure, porous nature, and functional construction units, porphyrin-based COFs have widespread applications in gas storage/separation [66][67], catalysis [68][69], sensing [70][71], energy storage [72][73], and phototherapy [74][75].
The construction of porphyrin-based COFs using porphyrins as the light-capturing units provides a unique solution for the stable and efficient porphyrin-based photocatalytic system and exhibits great potential in photocatalytic degradation of organic pollutants, hydrogen production, inhibition of bacteria, and other aspects [62][76][77][78][79]. Through molecular design and incorporation of special functional groups, porphyrin-based COFs can be used in biomedical fields such as photodynamic therapy and photothermal therapy, indicating great values of porphyrin-based COFs in the conversion and utilization of light.
2. Molecular Design of Porphyrin-Based COFs
As photocatalysts, light absorption capacity, separation, and transport of photogenerated charge carriers are important to their photocatalytic properties. In porphyrin-based COFs, porphyrin groups linked in the form of covalent bonds can improve their stability, crystallinity, and catalytic activity during photocatalysis [80]. In addition, appropriate orbital energy levels and band gaps can be obtained by regulating porphyrin units, organic ligands, and conjugate bonds. Therefore, porphyrin-based COFs will be expected to have both excellent light-harvesting ability and efficient charge separation and transport behavior. Interestingly, the introduction of a donor–acceptor (D–A) pair into the conjugated structures can further enhance light capture and facilitate the separation of photogenerated charges [80]. Two key issues should be considered during the design and synthesis of porphyrin-based COFs: the first is the design of the building blocks, namely the structure of the building blocks and connection mode. The second is the synthesis method, which determines if the desired frameworks can be obtained [81][82].
2.1. Construction of Porphyrin-Based COFs
The preparation of porphyrin-based COFs is similar to those of other COFs. Borate condensation reaction (Figure 1a), triazine condensation reaction (Figure 2a), and imine condensation reaction (Figure 3a) are often used to construct porphyrin-based COFs [83]. The involved reactions are reversible and thermodynamically controlled [84][85]. There are error-checking and self-healing processes during the formation of the reversible covalent linkages [86][87][88], resulting in ordered and thermodynamically stable porphyrin-based COFs [89][90]. Additionally, other reactions, including the Yamamoto homo-coupling reaction [91], cubic acid amino condensation reaction [92], and a pot of esterification reaction [93], had been used to prepare porphyrin-based COFs (Figure 4).
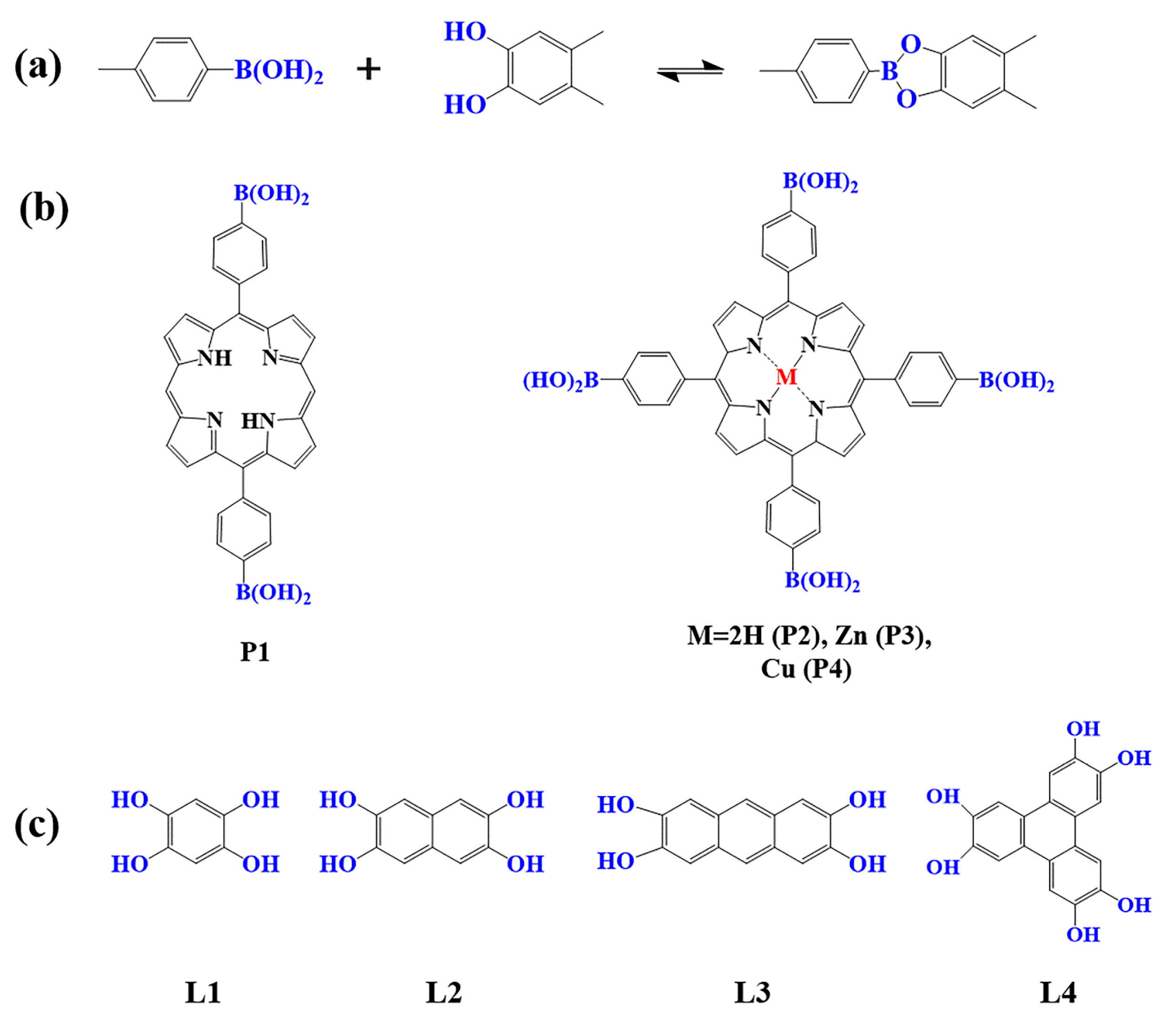
Figure 1. (a) Schematic illustration of borate-linked porphyrin-based COFs; (b) Frequently used monomers containing boron hydroxyl and (c) phenolic hydroxyl groups.
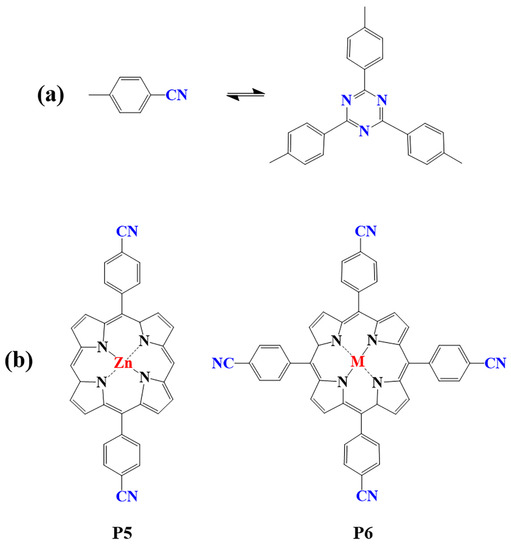
Figure 2. (a) Schematic illustration of triazine-linked porphyrin-based COFs; (b) Frequently used monomers.
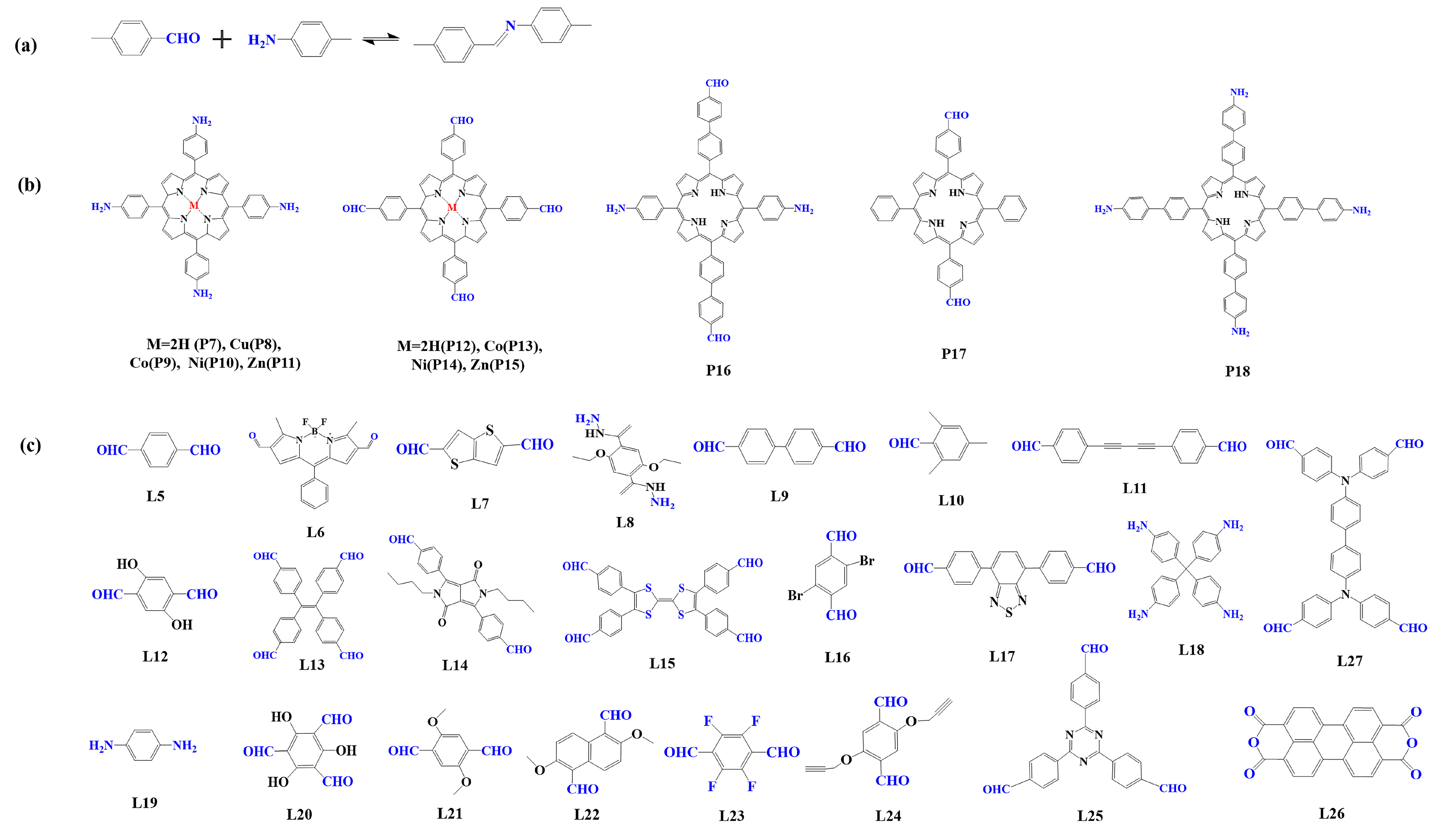
Figure 3. (a) Schematic illustration of imine-linked porphyrin-based COFs. (b) Frequently used monomers containing amino and (c) aldehyde groups.
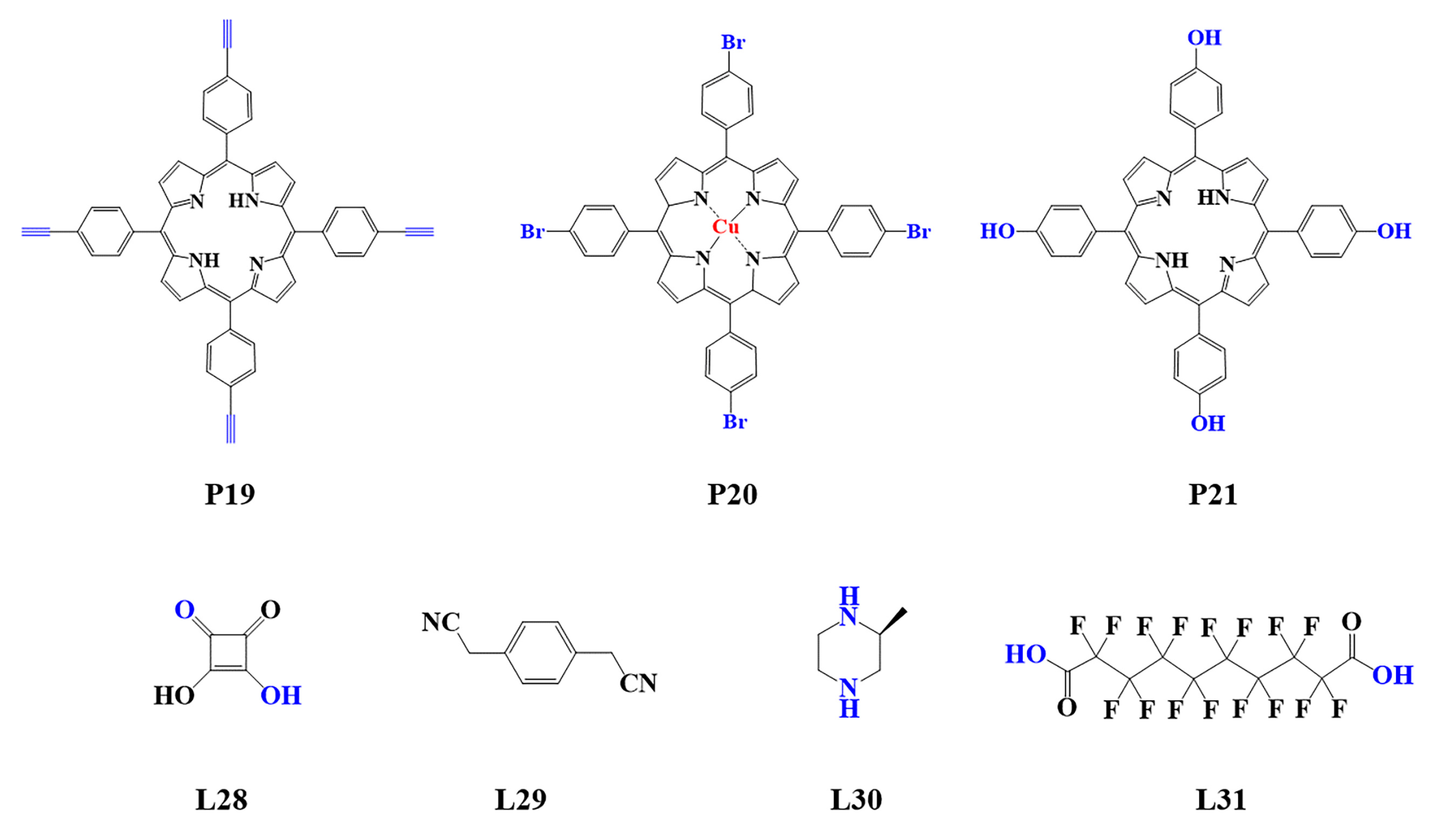
Figure 4. Monomers used in the synthesis of porphyrin-based COFs.
2.1.1. Construction of Borate-Linked Porphyrin-Based COFs
As a common reaction in organic synthesis, borate bonds generated from the condensation of borate acid and catechol derivatives are widely used to construct porphyrin-based COFs (Figure 1a). For example, porphyrin monomers containing p-dihydroxy boron (P1) and tetra-dihydroxy-beryl groups with different core structures (P2–P4) are condensed with 1, 2, 4, 5-tetrahydroxy benzene (L1–L4) to construct a series of borate porphyrin-based COFs [94][95][96][97]. Such porphyrin-based COFs with B–O linkages showed high crystallinity, special pore size, and large surface areas. However, compared to the porphyrin-based COFs linked by imine and triazine bonds, the COFs with borate-linkage are less stable in acidic and basic aqueous solutions, which limits their application in photocatalysis to some extent [83].
2.1.2. Construction of Triazine-Linked Porphyrin-Based COFs
Porphyrin-based covalent triazine frameworks are formed by cyclotrimerization of porphyrin monomers with nitrile groups (Figure 2a). The strong aromatic group in the triazine unit and ring fusion of C=N bonds endow them with high chemical stability. However, these porphyrin-based COFs are usually amorphous and lack long-range molecular orderings due to the nonplanar trimerization route [98]. In addition, only limited members of triazine porphyrin-based COFs were reported due to the lack of suitable monomers (P5, P6) [83][99][100][101][102][103].
2.1.3. Construction of Imine-Linked Porphyrin-Based COFs
Imine condensation reaction (Schiff base reaction) is the most widely used reaction in the synthesis of COFs, which is formed by the condensation of the amino group and aldehyde group (Figure 3a) [83]. The reaction is reversible, and it is easy to form a regular and ordered crystalline structure in COFs. In 2011, Yaghi et al. [95] synthesized the first imine bond-linked porphyrin COFs (COF-366) by the condensation of 5, 10, 15, 20-tetrad -(4-(amino) phenyl)-porphyrin (TAPP, P7) with p-phenyl formaldehyde (L5) under solvothermal condition, in which the carrier mobility reached 8.1 cm2 V−1 s−1. From then on, researchers prepared various imine bond-linked metal porphyrin-based COFs [104][105][106] by using different metal porphyrin monomers. The results showed that their photophysical and electronic properties could be regulated by the core metal ions. At the same time, to construct photocatalysts with high photocatalytic performance, researchers prefer to select monomers with electron-donating or accepting properties [62][107][108].
2.2. Synthesis of Porphyrin-Based COFs
The synthesis methods, structures, and properties of porphyrin-based COFs are dependent on the adopted preparation procedures. Till now, several synthetic methods, including solvothermal, microwave synthesis, and ionothermal methods, have been adopted to prepare porphyrin-based COFs. Among them, the solvothermal method is most widely used because of its high degree of universality [109]. Regular COFs were produced through the process of dissolution and recrystallization of raw materials in a closed pressure vessel at a certain temperature [86]. Great efforts have been made to optimize the synthesis conditions for the preparation of high-quality COFs. The crystallinity of porphyrin-based COFs is very sensitive to the reaction conditions. Therefore, their crystal state, morphology, pore size distribution, and other properties would be related to solvent, catalyst, temperature, reaction time, pressure, and other reaction parameters [110][111][112]. At the same time, the intrinsic relationship between the structure and property also attracted considerable attention and was usually used to direct the regulation of their photocatalytic properties [83][90].
Microwave synthesis showed several advantages over solvothermal methods, such as high reaction speed, high heading efficiency, and well-distributed heating [113], resulting in the formation of cleaner products with higher yields within less time. Compared to traditional thermal-assisted methods, microwave-assisted methods have the advantages of easy control, fast kinetic energy speed, high heating efficiency, and fast and uniform heating rate [114], which is conducive to obtaining COFs with high purity. The ionothermal method refers to the cyclization trimerization reaction in ZnCl2 at 400 °C using a nitrogen heterocyclic ring as the basic element [100]. The reaction is only partially reversible, resulting in less crystallinity. Moreover, harsh reaction conditions are usually required, which narrows the scope of porphyrin-based COFs.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics8020171
References
- Balzani, V.; Bergamini, G.; Ceroni, P. Photochemistry and photocatalysis. Rend. Lincei 2017, 28, 125–142.
- Banerjee, T.; Podjaski, F.; Kröger, J.; Biswal, B.P.; Lotsch, B.V. Polymer photocatalysts for solar-to-chemical energy conversion. Nat. Rev. Mater. 2020, 6, 168–190.
- Huang, C.W.; Nguyen, V.H.; Zhou, S.R.; Hsu, S.Y.; Tan, J.X.; Wu, K.C.W. Metal-organic frameworks: Preparation and applications in highly efficient heterogeneous photocatalysis. Sustain. Energ. Fuels 2020, 4, 504–521.
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985.
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38.
- Buzzetti, L.; Crisenza, G.E.; Melchiorre, P. Mechanistic studies in photocatalysis. Angew. Chem. Int. Edit. 2019, 58, 3730–3747.
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176.
- Corp, K.L.; Schlenker, C.W. Ultrafast spectroscopy reveals electron-transfer cascade that improves hydrogen evolution with carbon nitride photocatalysts. J. Am. Chem. Soc. 2017, 139, 7904–7912.
- Deponti, E.; Natali, M. Photocatalytic hydrogen evolution with ruthenium polypyridine sensitizers: Unveiling the key factors to improve efficiencies. Dalton Trans. 2016, 45, 9136–9147.
- Du, P.; Eisenberg, R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ. Sci. 2012, 5, 6012–6021.
- Eckenhoff, W.T.; McNamara, W.R.; Du, P.; Eisenberg, R. Cobalt complexes as artificial hydrogenases for the reductive side of water splitting. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 958–973.
- Ehrmaier, J.; Karsili, T.N.; Sobolewski, A.L.; Domcke, W. Mechanism of photocatalytic water splitting with graphitic carbon nitride: Photochemistry of the heptazine-water complex. J. Phys. Chem. Lett. 2017, 121, 4754–4764.
- Lau, V.W.H.; Yu, V.W.Z.; Ehrat, F.; Botari, T.; Moudrakovski, I.; Simon, T.; Duppel, V.; Medina, E.; Stolarczyk, J.K.; Feldmann, J.; et al. Urea-modified carbon nitrides: Enhancing photocatalytic hydrogen evolution by rational defect engineering. Adv. Energy Mater. 2017, 7, 1602251.
- Lau, V.W.-H.; Moudrakovski, I.; Botari, T.; Weinberger, S.; Mesch, M.B.; Duppel, V.; Senker, J.; Blum, V.; Lotsch, B.V. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 2016, 7, 12165.
- Meier, C.B.; Sprick, R.S.; Monti, A.; Guiglion, P.; Lee, J.S.M.; Zwijnenburg, M.A.; Cooper, A. Structure-property relationships for covalent triazine-based frameworks: The effect of spacer length on photocatalytic hydrogen evolution from water. Polymer 2017, 126, 283–290.
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329.
- Vyas, V.S.; Lau, V.W.H.; Lotsch, B.V. Soft photocatalysis: Organic polymers for solar fuel production. Chem. Mater. 2016, 28, 5191–5204.
- Wang, F.; Wang, W.G.; Wang, H.Y.; Si, G.; Tung, C.H.; Wu, L.Z. Artificial photosynthetic systems based on -hydrogenase mimics: The road to high efficiency for light-driven hydrogen evolution. ACS Catal. 2012, 2, 407–416.
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated polymers: Catalysts for photocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727.
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043.
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244.
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570.
- Wang, S.; Liu, F.; Ma, N.; Li, Y.; Jing, Q.; Zhou, X.; Xia, Y. Mechanistic process understanding of the self-assembling behavior of asymmetric bolaamphiphilic short-peptides and their templating for silica and titania nanomaterials. Nanoscale 2021, 13, 13318–13327.
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80.
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320.
- Zhang, G.; Liu, G.; Wang, L.; Irvine, J.T. Inorganic perovskite photocatalysts for solar energy utilization. Chem. Soc. Rev. 2016, 45, 5951–5984.
- Liu, F.; Cao, H.; Xu, L.; Fu, H.; Sun, S.; Xiao, Z.; Sun, C.; Long, X.; Xia, Y.; Wang, S. Design and preparation of highly active TiO2 photocatalysts by modulating their band structure. J. Colloid Interf. Sci. 2023, 629, 336–344.
- Kovaric, B.C.; Kokona, B.; Schwab, A.D.; Twomey, M.A.; de Paula, J.C.; Fairman, R. Self-Assembly of Peptide Porphyrin Complexes: Toward the Development of Smart Biomaterials. J. Am. Chem. Soc. 2006, 128, 4166–4167.
- Ng, K.K.; Lovell, J.F.; Vedadi, A.; Hajian, T.; Zheng, G. Self-Assembled Porphyrin Nanodiscs with Structure-Dependent Activation for Phototherapy and Photodiagnostic Applications. ACS Nano 2013, 7, 3484–3490.
- Panda, M.K.; Ladomenou, K.; Coutsolelos, A.G. Porphyrins in bio-inspired transformations: Light-harvesting to solar cell. Coord. Chem. Rev. 2012, 256, 2601–2627.
- Liu, K.; Xing, R.; Chen, C.; Shen, G.; Yan, L.; Zou, Q.; Ma, G.; Möhwald, H.; Yan, X. Peptide-Induced Hierarchical Long-Range Order and Photocatalytic Activity of Porphyrin Assemblies. Angew. Chem. Int. Ed. 2015, 127, 510–515.
- Patil, A.J.; Lee, Y.C.; Yang, J.W.; Mann, S. Mesoscale integration in titania/J-aggregate hybrid nanofibers. Angew. Chem. Int. Ed. 2012, 51, 733–737.
- Wang, S.; Li, M.; Patil, A.J.; Sun, S.; Tian, L.; Zhang, D.; Cao, M.; Mann, S. Design and construction of artificial photoresponsive protocells capable of converting day light to chemical energy. J. Mater. Chem. A 2017, 5, 24612–24616.
- Wang, L.; Fan, H.; Bai, F. Porphyrin-based photocatalysts for hydrogen production. MRS Bull. 2020, 45, 49–56.
- Nikoloudakis, E.; Karikis, K.; Han, J.; Kokotidou, C.; Charisiadis, A.; Folias, F.; Douvas, A.M.; Mitraki, A.; Charalambidis, G.; Yan, X. A self-assembly study of PNA-porphyrin and PNA-BODIPY hybrids in mixed solvent systems. Nanoscale 2019, 11, 3557–3566.
- Wang, J.; Zhong, Y.; Wang, L.; Zhang, N.; Cao, R.; Bian, K.; Alarid, L.; Haddad, R.E.; Bai, F.; Fan, H. Morphology-controlled synthesis and metalation of porphyrin nanoparticles with enhanced photocatalytic performance. Nano Lett. 2016, 16, 6523–6528.
- Zhong, Y.; Wang, J.; Zhang, R.; Wei, W.; Wang, H.; Lü, X.; Bai, F.; Wu, H.; Haddad, R.; Fan, H. Morphology-controlled self-assembly and synthesis of photocatalytic nanocrystals. Nano Lett. 2014, 14, 7175–7179.
- Wang, J.; Zhong, Y.; Wang, X.; Yang, W.; Bai, F.; Zhang, B.; Alarid, L.; Bian, K.; Fan, H. pH-dependent assembly of porphyrin-silica nanocomposites and their application in targeted photodynamic therapy. Nano Lett. 2017, 17, 6916–6921.
- Wang, D.; Niu, L.; Qiao, Z.-Y.; Cheng, D.-B.; Wang, J.; Zhong, Y.; Bai, F.; Wang, H.; Fan, H. Synthesis of self-assembled porphyrin nanoparticle photosensitizers. ACS Nano 2018, 12, 3796–3803.
- Devaramani, S.; Shinger, M.I.; Ma, X.; Yao, M.; Zhang, S.; Qin, D.; Lu, X. Porphyrin aggregates decorated MWCNT film for solar light harvesting: Influence of J- and H-aggregation on the charge recombination resistance, photocatalysis, and photoinduced charge transfer kinetics. Phys. Chem. Chem. Phys. 2017, 19, 18232–18242.
- Xiu, Y.; Zhang, X.; Feng, Y.; Wei, R.; Wang, S.; Xia, Y.; Cao, M.; Wang, S. Peptide-mediated porphyrin based hierarchical complexes for light-to-chemical conversion. Nanoscale 2020, 12, 15201–15208.
- Wang, S.; Zhang, D.; Zhang, X.; Yu, D.; Jiang, X.; Wang, Z.; Cao, M.; Xia, Y.; Liu, H. Short peptide-regulated aggregation of porphyrins for photoelectric conversion. Sustain. Energy Fuels 2019, 3, 529–538.
- Liu, K.; Yuan, C.; Zou, Q.; Xie, Z.; Yan, X. Self-Assembled Zinc/Cystine-Based Chloroplast Mimics Capable of Photoenzymatic Reactions for Sustainable Fuel Synthesis. Angew. Chem. Int. Ed. 2017, 56, 7876–7880.
- Xie, Z.; Liu, K.; Ren, X.; Zhang, H.; Xin, X.; Zou, Q.; Yan, X. Amino-Acid-Mediated Biomimetic Formation of Light-Harvesting Antenna Capable of Hydrogen Evolution. ACS Appl. Bio Mater. 2018, 1, 748–755.
- Han, J.J.; Liu, K.; Chang, R.; Zhao, L.Y.; Yan, X.H. Photooxidase-Mimicking Nanovesicles with Superior Photocatalytic Activity and Stability Based on Amphiphilic Amino Acid and Phthalocyanine Co-Assembly. Angew. Chem. Int. Ed. 2019, 58, 2000–2004.
- Xiu, Y.; Zhang, D.; Xu, L.; Li, J.; Chen, Y.; Xia, Y.; Cao, M.; Wang, S. Bioinspired construction of light-harvesting antenna via hierarchically co-assembling approach. J. Colloid Interf. Sci. 2021, 587, 550–560.
- Xiu, Y.; Xu, L.; Zhang, X.; Wang, X.; Liu, F.; Xia, Y.; Cao, M.; Wang, S. Mechanistic Process Understanding of the Biomimetic Construction of Porphyrin-Based Light-Capturing Antennas from Self-Assembled Fmoc-Blocked Peptide Templates. ACS Sustain. Chem. Eng. 2020, 8, 15761–15771.
- Li, L.L.; Yang, C.J.; Chen, W.H.; Lin, K.J. Towards the Development of Electrical Conduction and Lithium-Ion Transport in a Tetragonal Porphyrin Wire. ACS Sustain. Chem. Eng. 2003, 115, 1543–1546.
- Pasternack, R.F.; Gibbs, E.J. Porphyrin and metalloporphyrin interactions with nucleic acids. Met. Ions Biol. Syst. 1996, 33, 367.
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713.
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277.
- Yagai, S.; Seki, T.; Karatsu, T.; Kitamura, A.; Würthner, F. Transformation from H-to J-aggregated perylene bisimide dyes by complexation with cyanurates. Angew. Chem. Int. Ed. 2008, 47, 3367–3371.
- Purrello, R.; Monsu’Scolaro, L.; Bellacchio, E.; Gurrieri, S.; Romeo, A. Chiral H-and J-type aggregates of meso-tetrakis (4-sulfonatophenyl) porphine on α-helical polyglutamic acid induced by cationic porphyrins. Inorg. Chem. 1998, 37, 3647–3648.
- De Luca, G.; Romeo, A.; Scolaro, L.M. Aggregation properties of hyperporphyrins with hydroxyphenyl substituents. J. Phys. Chem. B 2006, 110, 14135–14141.
- Arai, Y.; Segawa, H. Cl-complexation induced H-and J-aggregation of meso-tetrakis (4-sulfonatothienyl) porphyrin diacid in aqueous solution. J. Phys. Chem. B 2011, 115, 7773–7780.
- Micali, N.; Villari, V.; Castriciano, M.A.; Romeo, A.; Monsù Scolaro, L. From fractal to nanorod porphyrin J-aggregates. Concentration-induced tuning of the aggregate size. J. Phys. Chem. B 2006, 110, 8289–8295.
- Castriciano, M.A.; Romeo, A.; Villari, V.; Micali, N.; Scolaro, L.M. Structural rearrangements in 5, 10, 15, 20-tetrakis (4-sulfonatophenyl) porphyrin J-aggregates under strongly acidic conditions. J. Phys. Chem. B 2003, 107, 8765–8771.
- Gandini, S.d.C.M.; Gelamo, E.L.; Itri, R.; Tabak, M. Small angle X-ray scattering study of meso-tetrakis (4-sulfonatophenyl) porphyrin in aqueous solution: A self-aggregation model. Biophys. J. 2003, 85, 1259–1268.
- Hollingsworth, J.V.; Richard, A.J.; Vicente, M.G.H.; Russo, P.S. Characterization of the Self-Assembly of meso-Tetra (4-sulfonatophenyl) porphyrin (H2TPPS4-) in Aqueous Solutions. Biomacromolecules 2012, 13, 60–72.
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal-Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444.
- Lin, S.; Diercks, C.S.; Zhang, Y.B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.B.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213.
- Zhang, L.; Yang, G.P.; Xiao, S.J.; Tan, Q.G.; Zheng, Q.Q.; Liang, R.P.; Qiu, J.D. Facile Construction of Covalent Organic Framework Nanozyme for Colorimetric Detection of Uranium. Small 2021, 17, e2102944.
- Zhang, L.; Wang, S.; Zhou, Y.; Wang, C.; Zhang, X.Z.; Deng, H. Covalent organic frameworks as favorable constructs for photodynamic therapy. Angew. Chem. Int. Ed. 2019, 58, 14213–14218.
- Li, H.; Liu, H.; Li, C.; Liu, J.; Liu, J.; Yang, Q.J. Micro-scale spatial location engineering of COF-TiO2 heterojunctions for visible light driven photocatalytic alcohol oxidation. J. Mater. Chem. A 2020, 8, 18745–18754.
- Lu, M.; Liu, J.; Li, Q.; Zhang, M.; Liu, M.; Wang, J.L.; Yuan, D.Q.; Lan, Y.Q. Rational Design of Crystalline Covalent Organic Frameworks for Efficient CO2 Photoreduction with H2O. Angew. Chem. Int. Ed. 2019, 58, 12392–12397.
- Li, Z.; Feng, X.; Zou, Y.; Zhang, Y.; Xia, H.; Liu, X.; Mu, Y. A 2D azine-linked covalent organic framework for gas storage applications. Chem. Commun. 2014, 50, 13825–13828.
- Das, P.; Mandal, S.K. In-depth experimental and computational investigations for remarkable gas/vapor sorption, selectivity, and affinity by a porous nitrogen-rich covalent organic framework. Chem. Mater. 2019, 31, 1584–1596.
- Ding, S.Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.G.; Su, C.Y.; Wang, W. Construction of covalent organic framework for catalysis: Pd/COF-LZU1 in Suzuki-Miyaura coupling reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822.
- Hou, J.; Zhang, H.; Simon, G.P.; Wang, H. Polycrystalline advanced microporous framework membranes for efficient separation of small molecules and ions. Adv. Mater. 2020, 32, 1902009.
- Wan, S.; Guo, J.; Kim, J.; Ihee, H.; Jiang, D. A belt-shaped, blue luminescent, and semiconducting covalent organic framework. Angew. Chem. 2008, 120, 8958–8962.
- Ascherl, L.; Evans, E.W.; Gorman, J.; Orsborne, S.; Bessinger, D.; Bein, T.; Friend, R.H.; Auras, F. Perylene-based covalent organic frameworks for acid vapor sensing. J. Am. Chem. Soc. 2019, 141, 15693–15699.
- DeBlase, C.R.; Silberstein, K.E.; Truong, T.T.; Abruña, H.D.; Dichtel, W.R. β-Ketoenamine-linked covalent organic frameworks capable of pseudocapacitive energy storage. J. Am. Chem. Soc. 2013, 135, 16821–16824.
- Vitaku, E.; Gannett, C.N.; Carpenter, K.L.; Shen, L.; Abruña, H.D.; Dichtel, W.R. Phenazine-based covalent organic framework cathode materials with high energy and power densities. J. Am. Chem. Soc. 2019, 142, 16–20.
- Fang, Q.; Wang, J.; Gu, S.; Kaspar, R.B.; Zhuang, Z.; Zheng, J.; Guo, H.; Qiu, S.; Yan, Y. 3D porous crystalline polyimide covalent organic frameworks for drug delivery. J. Am. Chem. Soc. 2015, 137, 8352–8355.
- Zhang, G.; Li, X.; Liao, Q.; Liu, Y.; Xi, K.; Huang, W.; Jia, X. Water-dispersible PEG-curcumin/amine-functionalized covalent organic framework nanocomposites as smart carriers for in vivo drug delivery. Nat. Commun. 2018, 9, 2785.
- Cui, W.R.; Zhang, C.R.; Xu, R.H.; Chen, X.R.; Yan, R.H.; Jiang, W.; Liang, R.P.; Qiu, J.D. Low Band Gap Benzoxazole-Linked Covalent Organic Frameworks for Photo-Enhanced Targeted Uranium Recovery. Small 2021, 17, 2006882.
- Cheng, C.; He, B.; Fan, J.; Cheng, B.; Cao, S.; Yu, J. An inorganic/organic S-scheme heterojunction H2-production photocatalyst and its charge transfer mechanism. Adv. Mater. 2021, 33, 2100317.
- Khaing, K.K.; Yin, D.; Ouyang, Y.; Xiao, S.; Liu, B.; Deng, L.; Li, L.; Guo, X.; Wang, J.; Liu, J. Fabrication of 2D-2D heterojunction catalyst with covalent organic framework (COF) and MoS2 for highly efficient photocatalytic degradation of organic pollutants. Inorg. Chem. 2020, 59, 6942–6952.
- Lin, G.; Ding, H.; Chen, R.; Peng, Z.; Wang, B.; Wang, C. 3D porphyrin-based covalent organic frameworks. J. Am. Chem. Soc. 2017, 139, 8705–8709.
- Li, H.; Wang, L.; Yu, G. Covalent organic frameworks: Design, synthesis, and performance for photocatalytic applications. Nano Today 2021, 40, 101247.
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
- Lv, N.; Li, Q.; Zhu, H.; Mu, S.; Luo, X.; Ren, X.; Liu, X.; Li, S.; Cheng, C.; Ma, T. Electrocatalytic Porphyrin/Phthalocyanine-Based Organic Frameworks: Building Blocks, Coordination Microenvironments, Structure-Performance Relationships. Adv. Sci. 2023, 10, 2206239.
- Huang, S.; Chen, K.; Li, T.T. Porphyrin and phthalocyanine based covalent organic frameworks for electrocatalysis. Coordin. Chem. Rev. 2022, 464, 214563.
- Rodríguez-San-Miguel, D.; Montoro, C.; Zamora, F. Covalent organic framework nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2020, 49, 2291–2302.
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M. Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem. Soc. Rev. 2019, 48, 5266–5302.
- Cote, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170.
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed synthesis of 3D covalent organic frameworks. Science 2007, 316, 268–272.
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714.
- Wilson, A.; Gasparini, G.; Matile, S. Functional systems with orthogonal dynamic covalent bonds. Chem. Soc. Rev. 2014, 43, 1948–1962.
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.; Sanders, J.K.; Stoddart, J.F. Dynamic covalent chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952.
- Wang, X.S.; Liu, J.; Bonefont, J.M.; Yuan, D.Q.; Thallapally, P.K.; Ma, S. A porous covalent porphyrin framework with exceptional uptake capacity of saturated hydrocarbons for oil spill cleanup. Chem. Commun. 2013, 49, 1533–1535.
- Nagai, A.; Chen, X.; Feng, X.; Ding, X.; Guo, Z.; Jiang, D. A squaraine-linked mesoporous covalent organic framework. Angew. Chem. Int. Ed. 2013, 52, 3770–3774.
- Tao, D.; Feng, L.; Chao, Y.; Liang, C.; Song, X.; Wang, H.; Yang, K.; Liu, Z. Covalent Organic Polymers Based on Fluorinated Porphyrin as Oxygen Nanoshuttles for Tumor Hypoxia Relief and Enhanced Photodynamic Therapy. Adv. Funct. Mater. 2018, 28, 1804901.
- Feng, X.; Liu, L.; Honsho, Y.; Saeki, A.; Seki, S.; Irle, S.; Dong, Y.; Nagai, A.; Jiang, D. High-rate charge-carrier transport in porphyrin covalent organic frameworks: Switching from hole to electron to ambipolar conduction. Angew. Chem. Int. Ed. 2012, 51, 2618–2622.
- Wan, S.; Gándara, F.; Asano, A.; Furukawa, H.; Saeki, A.; Dey, S.K.; Liao, L.; Ambrogio, M.W.; Botros, Y.Y.; Duan, X.; et al. Covalent Organic Frameworks with High Charge Carrier Mobility. Chem. Mater. 2011, 23, 4094–4097.
- Park, S.; Liao, Z.; Ibarlucea, B.; Qi, H.; Lin, H.H.; Becker, D.; Melidonie, J.; Zhang, T.; Sahabudeen, H.; Baraban, L. Two-dimensional boronate ester covalent organic framework thin films with large single crystalline domains for a neuromorphic memory device. Angew. Chem. 2020, 132, 8295–8301.
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Löbermann, F.; Trauner, D. Extraction of photogenerated electrons and holes from a covalent organic framework integrated heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807.
- Liu, X.; Qi, R.; Li, S.; Liu, W.; Yu, Y.; Wang, J.; Wu, S.; Ding, K.; Yu, Y. Triazine-Porphyrin-Based Hyperconjugated Covalent Organic Framework for High-Performance Photocatalysis. J. Am. Chem. Soc. 2022, 144, 23396–23404.
- Ren, H.; Ben, T.; Wang, E.; Jing, X.; Xue, M.; Liu, B.; Cui, Y.; Qiu, S.; Zhu, G. Targeted synthesis of a 3D porous aromatic framework for selective sorption of benzene. Chem. Commun. 2010, 46, 291–293.
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453.
- Bojdys, M.J.; Jeromenok, J.; Thomas, A.; Antonietti, M. Rational extension of the family of layered, covalent, triazine-based frameworks with regular porosity. Adv. Mater. 2010, 22, 2202–2205.
- Hou, Y.; Huang, Y.B.; Liang, Y.L.; Chai, G.L.; Yi, J.D.; Zhang, T.; Zang, K.T.; Luo, J.; Xu, R.; Lin, H. Unraveling the reactivity and selectivity of atomically isolated metal-nitrogen sites anchored on porphyrinic triazine frameworks for electroreduction of CO2. Chem. Commun. 2019, 1, 384–395.
- Yi, J.-D.; Xu, R.; Chai, G.-L.; Zhang, T.; Zang, K.; Nan, B.; Lin, H.; Liang, Y.-L.; Lv, J.; Luo, J.; et al. Cobalt single-atoms anchored on porphyrinic triazine-based frameworks as bifunctional electrocatalysts for oxygen reduction and hydrogen evolution reactions. J. Mater. Chem. A 2019, 7, 1252–1259.
- Lv, H.; Sa, R.; Li, P.; Yuan, D.; Wang, X.; Wang, R. Metalloporphyrin-based covalent organic frameworks composed of the electron donor-acceptor dyads for visible-light-driven selective CO2 reduction. Sci. China Chem. 2020, 63, 1289–1294.
- Wu, Q.; Mao, M.J.; Wu, Q.J.; Liang, J.; Huang, Y.B.; Cao, R. Construction of Donor-Acceptor Heterojunctions in Covalent Organic Framework for Enhanced CO2 Electroreduction. Small 2021, 17, e2004933.
- Chen, R.; Wang, Y.; Ma, Y.; Mal, A.; Gao, X.Y.; Gao, L.; Qiao, L.; Li, X.B.; Wu, L.Z.; Wang, C. Rational design of isostructural 2D porphyrin-based covalent organic frameworks for tunable photocatalytic hydrogen evolution. Nat. Commun. 2021, 12, 1354.
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511.
- Jin, S.; Ding, X.; Feng, X.; Supur, M.; Furukawa, K.; Takahashi, S.; Addicoat, M.; El-Khouly, M.E.; Nakamura, T.; Irle, S. Charge dynamics in a donor-acceptor covalent organic framework with periodically ordered bicontinuous heterojunctions. Angew. Chem. Int. Ed. 2013, 7, 2017–2021.
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent organic frameworks: Design, synthesis, and functions. Chem. Rev. 2020, 120, 8814–8933.
- Smith, B.J.; Overholts, A.C.; Hwang, N.; Dichtel, W.R. Insight into the crystallization of amorphous imine-linked polymer networks to 2D covalent organic frameworks. Chem. Commun. 2016, 52, 3690–3693.
- Gao, Q.; Bai, L.; Zeng, Y.; Wang, P.; Zhang, X.; Zou, R.; Zhao, Y. Reconstruction of covalent organic frameworks by dynamic equilibrium. Chem. Eur. J. 2015, 21, 16818–16822.
- Feng, X.; Chen, L.; Dong, Y.; Jiang, D. Porphyrin-based two-dimensional covalent organic frameworks: Synchronized synthetic control of macroscopic structures and pore parameters. Chem. Commun. 2011, 47, 1979–1981.
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284.
- Zhou, S.; Meng, T.; Hu, D.; Zhu, Y.; Huang, C.; Song, M.; Gao, S.; Zhang, G. Characteristic Synthesis of a Covalent Organic Framework and Its Application in Multifunctional Tumor Therapy. ACS Appl. Bio Mater. 2022, 5, 59–81.
This entry is offline, you can click here to edit this entry!
