Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The guanylyl cyclase (sGC) is heme-containing enzyme, which causes an increase in the level of cyclic 3′-5′-guanosine monophosphate (cGMP) in smooth muscle and subsequent vascular relaxation.
- guanylate cyclase (GC)
- chronic heart failure (CHF)
- pulmonary arterial hypertension (PAH)
1. Introduction
In 2012, at the annual meeting of the American College of Chest Physicians (ACCP) in Atlanta, the results of two trials, CHEST-1 and PATENT-1, were presented, confirming the efficacy of riociguat in the treatment of chronic thromboembolic pulmonary hypertension (CTEPH) [1][2]. In addition, in 2021, the FDA approved the use of vericiguat for the treatment of heart failure. Research into the use of other sGC activators—including cinaciguat or pralinciguat—is still ongoing.
Nitric Oxide Pathway as a Target for Pharmacological Intervention in Pulmonary Arterial Hypertension and Heart Failure
There is a common element in the pathogenesis of diseases, such as heart failure and pulmonary arterial hypertension. It is the process of the hypertrophy and remodeling of the vascular wall coexisting with progressive fibrosis. The dysfunction of the vascular endothelium is of great importance in this process. The pharmacotherapy of both diseases is aimed at intervening in the pathways involved in the influence of mediators [3].
The impact of nitric oxide donors is short-lived due to the time-limited possibilities of the enzymatic release of nitric oxide, as well as the toxicity of some drugs. For this reason, research on the next links in the mechanism of nitric oxide or other pathways leading to an increase in the concentration of the key link—cGMP—has become so important [4][5].
The modern pharmacotherapy of heart failure includes converting enzyme inhibitors or neprilysin inhibitors, mineralocorticoid receptor antagonists, beta-adrenoreceptor antagonists, and sodium–glucose transport protein type 2 inhibitors. Among the drugs included in the next steps are also guanylate cyclase activators. The pharmacotherapy of pulmonary arterial hypertension includes prostacyclin analogs, phosphodiesterase-5 inhibitors, endothelin-1 receptor antagonists, and a modulator of guanylate cyclase activity. During the therapeutic effect of the above-mentioned groups of drugs, an increase in cGMP concentration is obtained directly or indirectly [3][4][5]. Angiotensin converting enzyme inhibitors, by inhibiting the degradation of bradykinin, increase its concentration. A similar effect occurs with the use of neprilysin inhibitors, but in addition to inhibiting the degradation of bradykinin, the degradation of natriuretic peptides is also inhibited. Phosphodiesterase-5 inhibitors inhibit cGMP degradation. In the case of beta-adrenoceptor antagonists, mineralocorticoid receptors, or sodium–glucose inhibitors of protein transport type 2, there is also an indirect relationship [5][6][7].
The idea of the direct modulation or stimulation of guanylate cyclase seemed promising from a theoretical point of view; moreover, clinical trials confirmed the efficacy in these clinical settings. The main goal was to increase the activity of the enzyme and thus achieve a synergistic effect between the applied pharmacotherapy and the activation of guanylate cyclase [5][8][9].
2. The Action of sGC Activators
Nitric oxide (NO) is an endogenous vasodilator synthesized by nitric oxide synthase (eNOS, NOS III, and NOS3) and released constantly from endothelial cells. Nitric oxide synthase exists in three isoforms, named after the tissue from which the enzyme was isolated initially: neuronal (NOS I, also called nNOS, NOS1), endothelial (eNOS, NOS3, NOS III), and inducible (iNOS, NOS2 NOS II). NOS1 is responsible for the transmission of nerve impulses, while NOS3 plays a role mainly in the vasodilation and regulation of arterial pressure [3].
NO signaling has pleiotropic roles In biology and a crucial function in cardiovascular homeostasis. An increase in NO secretion occurs under the influence of mediators, such as norepinephrine (NA), angiotensin, adenosine triphosphate, or bradykinin. NO synthesis is also stimulated as a result of numerous physical factors., e.g., increasing mechanical pressure and the pressure exerted on the endothelium by the flowing bloodstream or low oxygen partial pressure in the blood vessels [3][4][5].
The mechanism of NO’s intracellular action is primarily through the stimulation of the activity of the soluble form of guanylyl cyclase (sGC). The sGC is heme-containing enzyme, which causes an increase in the level of cyclic 3′-5′-guanosine monophosphate (cGMP) in smooth muscle and subsequent vascular relaxation [6]. For years, guanylate cyclase was thought to be a homogeneous, tissue-unspecific enzyme, but studies have shown that there are two main types of guanylate cyclase, which have different localization within the cell. The first is a membrane-bound cyclase (mGC), while the second is an entirely intracellular soluble guanylate cyclase (sGC). For the former, the agonists are peptides (natriuretic peptides A, B, C), and for the latter, they are gaseous mediators (nitric oxide, carbon monoxide).
NO/sGC/cGMP regulation plays a leading role in the homeostasis of the cardiovascular and pulmonary systems and organs, such as the kidney, brain, and liver. In addition to smooth muscle cells, cGMP influences the function of fibroblasts, cardiomyocytes, platelets, neurons, and immune cells, regulating the processes of fibrosis, inflammatory response, and neurotransmission [3][4][5][7] (Figure 1).
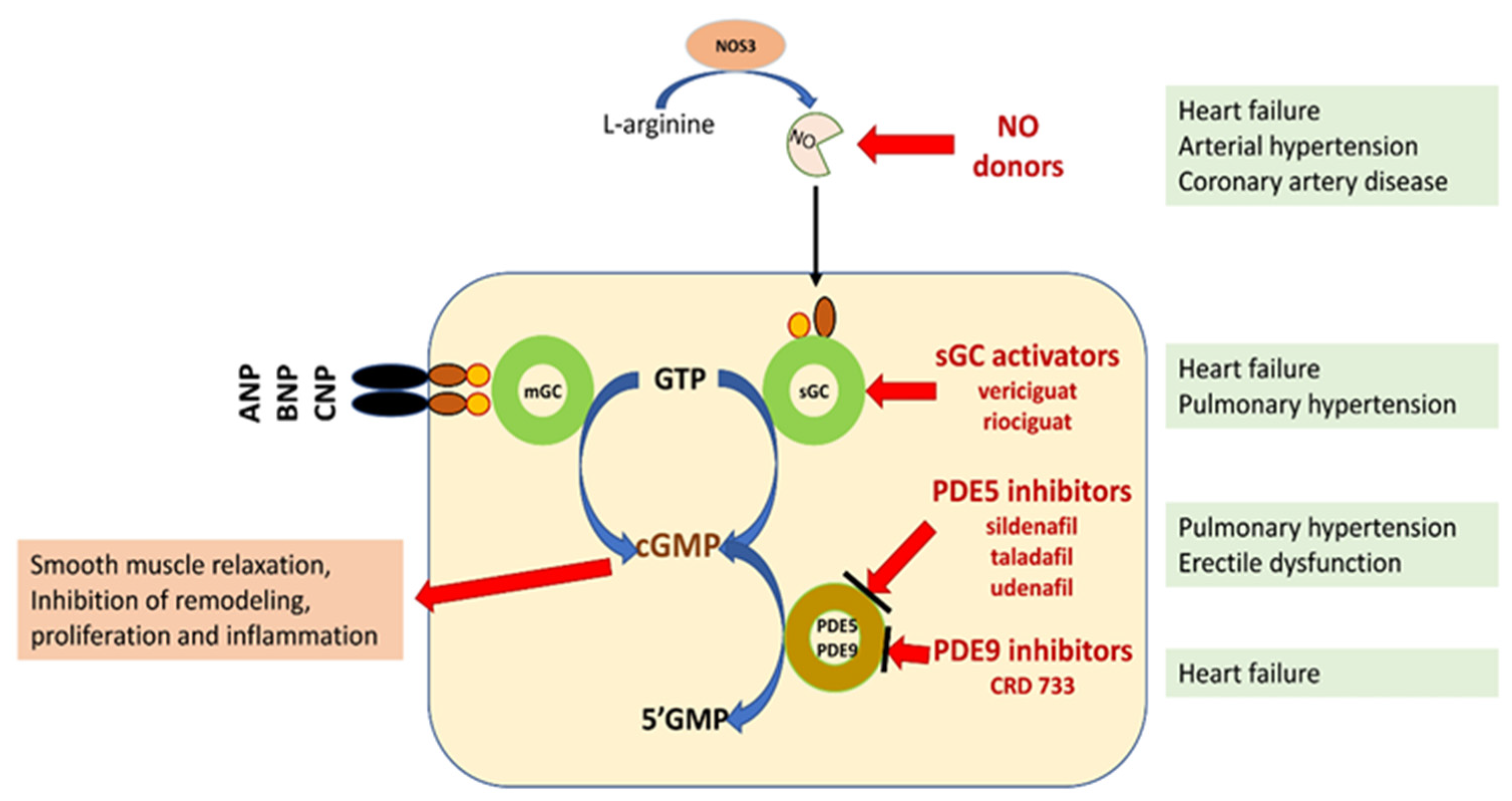
Figure 1. Schematic representation of the potential for pharmacological interference with the NO/sGC/cGMP pathway [3][5][6][7]. Explanation of abbreviations: sGC—soluble form of guanylyl cyclase; mGC—membrane form of guanylyl cyclase; NO—nitric oxide; eNOS—nitric oxide synthase; ANP, BNP, CMP—natriuretic peptide A, B or C; GMP 3′-5′—guanosine monophosphate; GTP 3′-5′—guanosine triphosphate; cGMP cyclic 3′-5′—guanosine monophosphate; CNGCs—cyclic nucleotide gated channels; PKG—protein kinase G; PDE phosphodiesterase.
The discovery of new classes of compounds, the so called sGC stimulators and sGC activators, or in medical lingo guates, that stimulate cGMP formation provided a tool to study sGC redox regulation and its role in pathogenesis. It also made it possible to target drugs directly at diseased blood vessels, heart muscle, kidneys, and other organs. From a pharmacological point of view, sGC stimulators increase the activity of sGC independently of NO and also act synergistically with endogenous NO. In contrast, sGC activators specifically bind to and activate an oxidized, heme-free form of sGC [8][9] (Table 1).
| Compound | Clinical Setting | Clinical Trial |
|---|---|---|
| riociguat | CTEPH | CHEST |
| riociguat | PAH, CTEPH, childhood PAH, erectile dysfunction | PATENT 2, CHEST 2, PATENT CHILD |
| vericiguat | HFrEF | SOCRATES-REDUCED |
| vericiguat | HF | SOCRATES -PRESERVED VICTORIA HFrEF, VITALITY HFpEF |
| praliciguat | HFpEF | CAPACITY -HFpEF |
| praliciguat | Diabetic neuropathy, sickle cell anemia, HF | STRONG SCD |
| oliciguat | Achalasia, sickle cell anemia | |
| cinaciguat | HF, acute decompensated HF | |
| Other compounds—potential clinical indications | Prostate hypertrophy, Reynaud’s syndrome in systemic sclerosis, cystic fibrosis, Duchenne muscular dystrophy, congenital bone fragility, non-alcoholic fatty liver disease, dementia, neuropathic pain, peripheral artery disease |
The sGC stimulators described to date, including lificiguat, riociguat, ataciguat, and cinaciguat, bind directly to sCG. In addition, riociguat was found to sensitize sGC to endogenous nitric oxide by stabilizing the nitric oxide–sGC bond [10][11][12].
3. Molecular Structures of the sCG Active Compounds
The molecular formulas of the sCG active compounds are gathered in Figure 2.
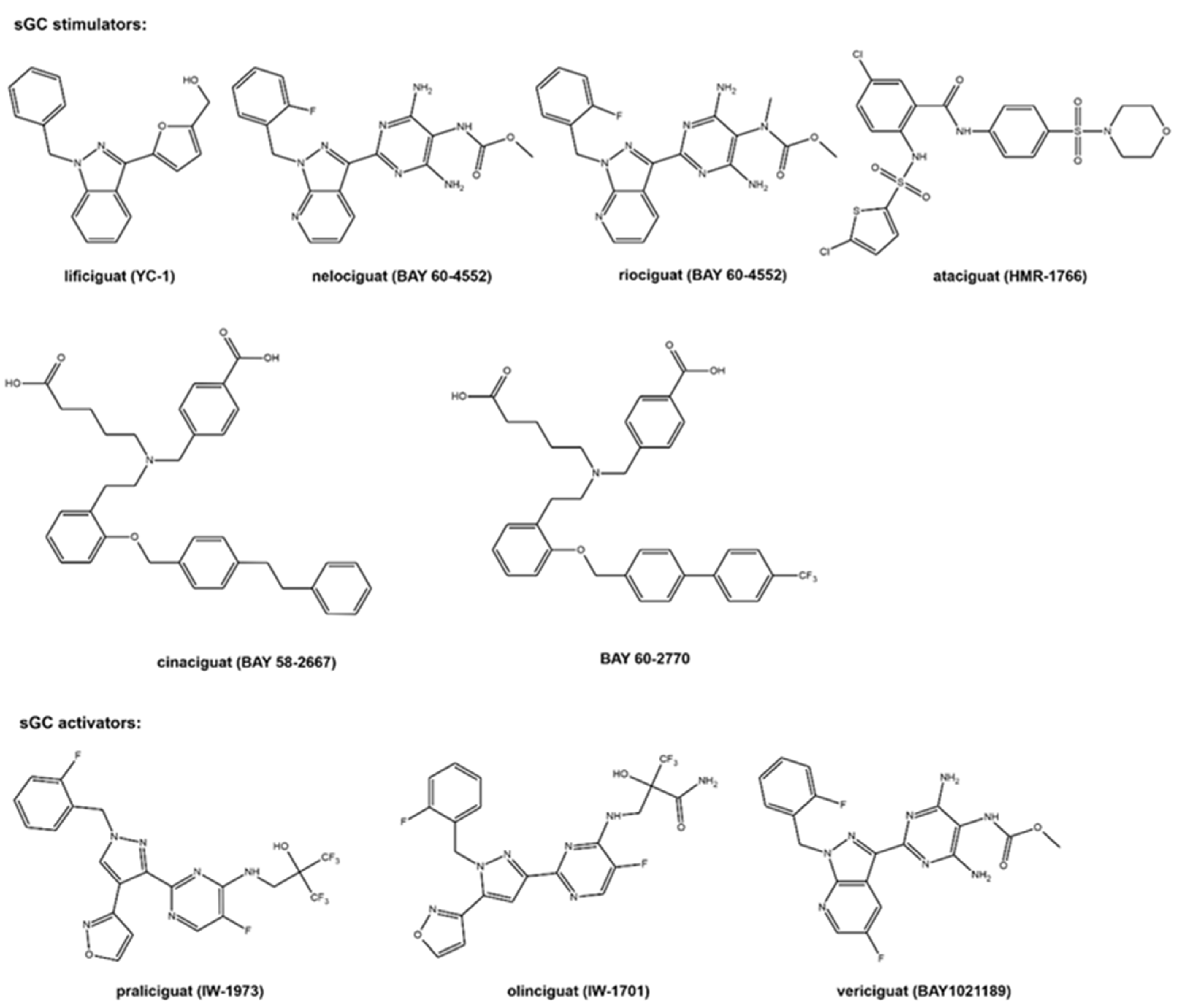
Figure 2. The molecular structure of the studied sCG compounds.
The first discovered sGC active compound was lificiguat (YC-1). From a chemical point of view, lificiguat is an indazole derivative whose structure contains furyl and benzyl substituents at positions 1 and 3, respectively. This benzylindazole compound does not exist in the nature and is obtained only by chemical synthesis [13] (Figure 2). The class of substances that was developed based on the modification of the structure of lificiguat originated from nelociguat. This group of substances includes pyridine pyrimidinopyrazole derivatives as the leading structure [13] Nelociguat can be classified as pyrazolopyridine, aminopyrimidine, organofluorine, and carbamate ester. The addition of one methyl group to the carbamate ester moiety results in a riociguat structure. Riociguat is the first compound in the sGC activator class approved as a drug [14] (Figure 2). It is worth noting that vericiguat (sGC activators, Figure 2) is an analog of the structure of nelociguat. The vericiguat structure has one additional fluoro substituent on the 1H-pyrazolo [3,4-b] pyridine moiety. SAR studies revealed that the introduction of an additional fluorine atom in the nelociguat changed the nature of the activity of these molecules from the stimulators to the activator of sGC [15].
Ataciguat is the anthranilic acid derivatives member. It stimulated sGC in a concentration-dependent and reversible fashion (EC50 of 0.51 μM and was active in vascular smooth muscle cells exposed to oxidative stress) [16]. Cinaciguat and BAY 60-2770 are examples of compounds that prove that an active sGC compound cannot contain a heterocyclic ring in the structure (Figure 2). Praliciguat, oliciguat, and vericiguat are shown as examples of sGC activators.
Apart from efficacy and toxicity, many drug development failures are imputable to poor pharmacokinetics and bioavailability. Bioavailability can be described by six physicochemical properties indices, such as lipophilicity, size, polarity, solubility, flexibility and saturation. [17]. Figure 3 shows the assessment of the similarity of drugs of the discussed compounds to known drugs in the form of bioavailability radar [18]. The presented comparison shows that the basic properties of the stimulators/activators synthesized so far differ from the known drugs [19]. This indicates some complex bioavailability process and the need for the careful selection of the dose of the preparations used.
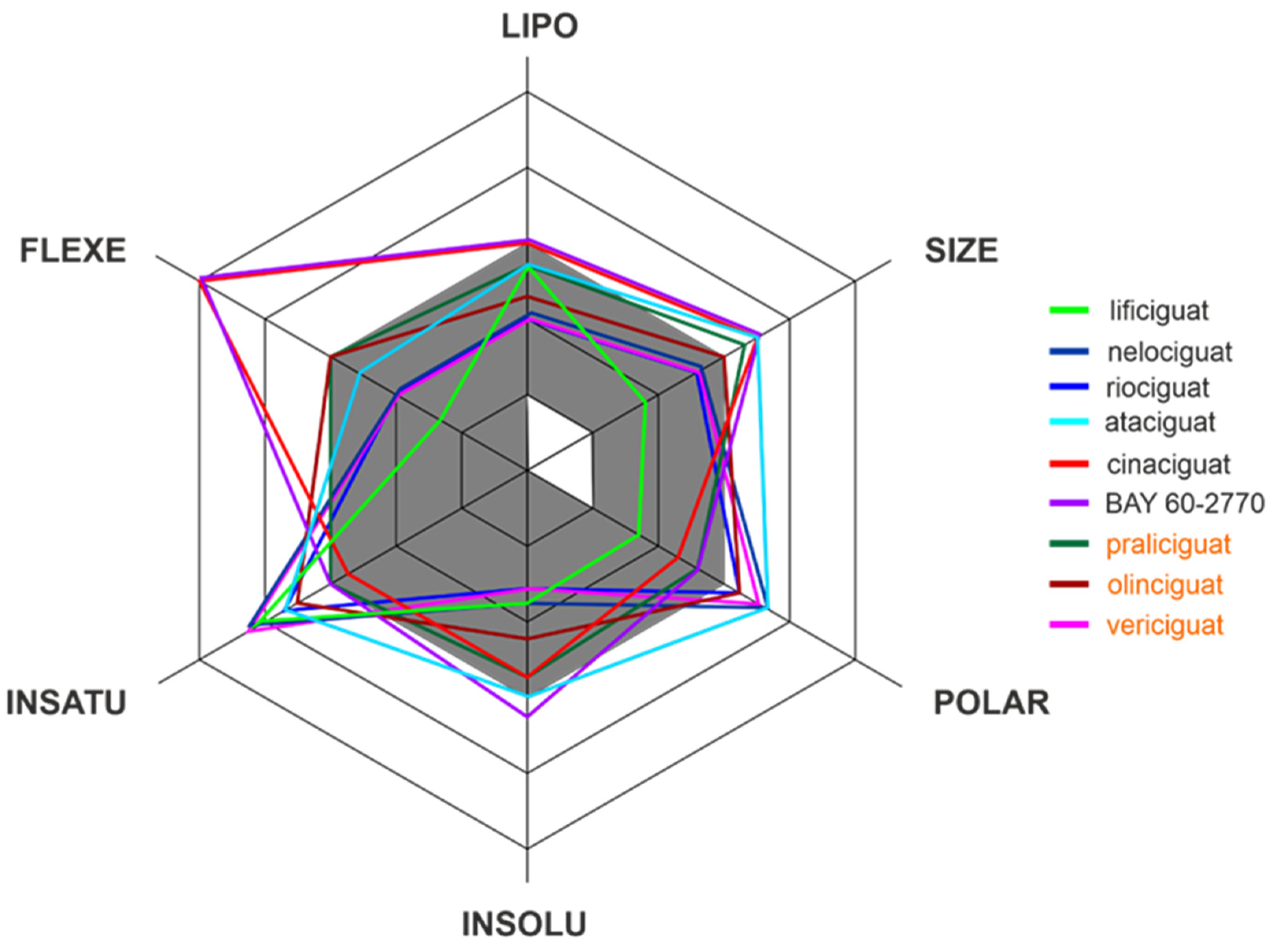
Figure 3. The bioavailability radar of the sCG activators/stimulators. The gray area represents the optimal range for each of the properties (lipophilicity: XLOGP3 between −0.7 and +5.0, size: MW between 150 and 500 g/mol, polarity: TPSA between 20 and 130 Å2, solubility: log S no higher than 6, saturation: fraction of carbons in the sp3 hybridization no less than 0.25, and flexibility: no more than 9 rotatable bonds). The compounds names are indicated in the different font colors, i.e., black corresponds to sGC stimulators and orange corresponds to sGC activators.
Of the various routes of drug administration, oral dosing of the drug is highly preferred. Therefore, the early estimation of oral bioavailability and the fraction of the dose that reaches the blood after oral administration is crucial in the drug discovery process. Nevertheless, bioavailability is highly multifactorial but depends mainly on absorption from the gastrointestinal tract, including metabolism in the gut wall [20]. The routinely applied prediction tool is the Brain Or IntestinaL EstimatED permeation method (BOILED-Egg, also called the Egan egg) proposed by Egan et al. [21] illustrate the gastrointestinal absorption (HIA) and brain penetration (BBB). The pharmacokinetic properties of the discussed compounds using the boiled egg model allows for the intuitive evaluation of passive gastrointestinal HIA and BBB in the function of the position of the molecules in the Log P versus TPSA referential (Figure 4). BOILED-EGG is fast and acceptable as an accurate predictive model (with an internal accuracy above 93 %) for pharmacokinetics and bioavailability drug behaviors. The white region is for high probability of passive absorption by the gastrointestinal tract, and the yellow region (yolk) is for high probability of brain penetration. Yolk and white areas are not mutually exclusive [22].
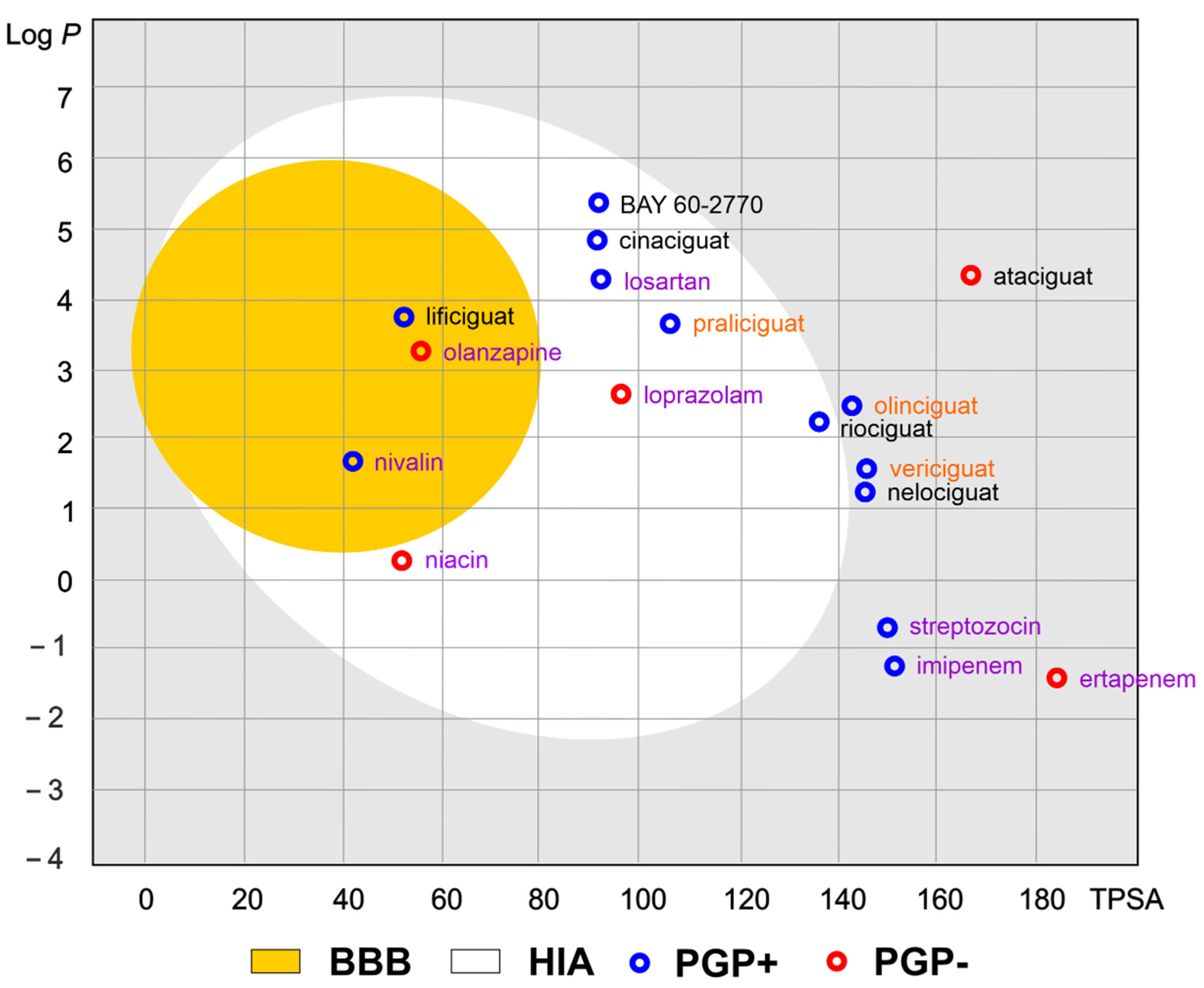
Figure 4. The Brain Or IntestinaL EstimatED (BOILED-Egg) graph compared sGC and selected commonly use drugs. The colored areas represent the optimal prediction range (above 93%) of penetration for brain (BBB, yellow) and gastrointestinal tract (HIA, white). The gray color indicates the area where penetration into the brain and gastrointestinal tract may occur but its probability is below the optimal value. The molecule predicted to be evaluated from the central nervous system by the P-glycoprotein (blue dots) or not (red dots). The compounds names are indicated in the different font colors, i.e., black corresponds to sGC stimulators, orange corresponds to sGC activators, and purple corresponds to selected drugs.
As shown in Figure 4, the values of the obtained physicochemical parameters for lificiguat indicate a high probability of penetration into the brain. In contrast, BAY 60-2770, cinaciguat, riociguat, and praliciguat have a high probability of penetrating the blood from the gastrointestinal tract.
This entry is adapted from the peer-reviewed paper 10.3390/molecules28020861
References
- Leber, L.; Beaudet, A.; Muller, A. Epidemiology of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Identification of the most accurate estimates from a systematic literature review. Pulm Circ. 2021, 11, 2045894020977300.
- Simonneau, G.; Gatzoulis, M.A.; Adatia, I.; Celermajer, D.; Denton, C.; Ghofrani, A.; Gomez Sanchez, M.A.; Krishna Kumar, R.; Landzberg, M.; Machado, R.F.; et al. Updated clinical classification of pulmonary hypertension. J. Am. Coll. Cardiol. 2013, 62, D34–D41.
- Nowaczyk, A.; Kowalska, M.; Nowaczyk, J.; Grześk, G. Carbon Monoxide and Nitric Oxide as the Examples of the Youngest Class of Transmitters. Int. J. Mol. Sci. 2021, 22, 6029.
- Derbyshire, E.R.; Marletta, M.A. Biochemistry of soluble guanylate cyclase. Handb. Exp. Pharmacol. 2009, 191, 17–31.
- Grześk, G.; Nowaczyk, A. Current modulation of guanylate cyclase pathway activity-mechanism and clinical implications. Molecules 2021, 26, 3418.
- Farah, C.; Michel, L.Y.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316.
- Jacuś, B.; Kowalkowska, M.; Miękus, P.; Grześk, G. Vericiguat jako stymulator cyklazy guanylanowej elementem innowacyjnej terapii niewydolności serca. Farm. Pol. 2021, 77, 615–621.
- Sandner, P.; Follmann, M.; Becker-Pelster, E.; Hahn, M.G.; Meier, C.; Freitas, C.; Roessig, L.; Stasch, J.P. Soluble GC stimulators and activators: Past, present and future. Br. J. Pharmacol. 2021; ahead of print.
- Sandner, P.; Stasch, J.P. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir. Med. 2017, 122 (Suppl. 1), S1–S9.
- Stasch, J.P.; Pacher, P.; Evgenov, O.V. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011, 123, 2263–2273.
- Sandner, P.; Zimmer, D.P.; Milne, G.T. Correction to: Soluble guanylate cyclase stimulators and activators. In Reactive Oxygen Species. Handbook of Experimental Pharmacology, vol 264; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer: Cham, Switzerland, 2019.
- Sandner, P.; Vakalopoulos, A.; Hahn, M.G.; Stasch, J.P.; Follman, M. Soluble guanylate cyclase stimulators and their potential use: A patent review. Expert Opin. Ther. Pat. 2021, 31, 203–222.
- Yu, K.H.; Hung, H.Y. Synthetic strategy and structure–activity relationship (SAR) studies of 3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1, Lificiguat): A review. RSC Adv. 2022, 12, 251–264.
- Zhou, X.; Hu, X.; Gu, J.; Zhu, J. Comparison of the crystal structures and thermochemistry of a novel soluble guanylate cyclase stimulator riociguat and its solvates. Acta Cryst. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 891–898.
- He, J.; Li, Z.; Dhawan, G.; Zhang, W.; Sorochinsky, A.E.; Butler, G.; Soloshonok, V.A.; Han, J. Fluorine-containing drugs approved by the FDA in 2021. Chin. Chem. Lett. 2022, 34, 107578.
- Schantl, A.E.; Ivarsson, M.E.; Leroux, J.-C. Investigational pharmacological treatments for vascular calcification. Adv. Therap. 2019, 2, 1800094.
- Bragina, M.E.; Daina, A.; Perez, M.A.S.; Michielin, O.; Zoete, V. The Swiss Similarity 2021 Web Tool: Novel Chemical Libraries and Additional Methods for an Enhanced Ligand-Based Virtual Screening Experience. Int. J. Mol. Sci. 2022, 23, 811.
- Ritchie, T.J.; Ertl, P.; Lewis, R. The graphical representation of ADME-related molecule properties for medicinal chemists. Drug. Discov. Today 2011, 16, 65–72.
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717.
- Grześk, G.; Rogowicz, D.; Wołowiec, Ł.; Ratajczak, A.; Gilewski, W.; Chudzińska, M.; Sinkiewicz, A.; Banach, J. The clinical significance of drug-food interactions of direct oral anticoagulants. Int. J. Mol. Sci. 2021, 22, 8531.
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877.
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121.
This entry is offline, you can click here to edit this entry!
