Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Materials Science, Biomaterials
Oral health is crucial to daily life, yet many people worldwide suffer from oral diseases. With the development of oral tissue engineering, there is a growing demand for dental biomaterials. Addressing oral diseases often requires a two-fold approach: fighting bacterial infections and promoting tissue growth. Hydrogels are promising tissue engineering biomaterials that show great potential for oral tissue regeneration and drug delivery.
- tissue engineering
- hydrogels
- dental pulp
- periodontium
- mandible
1. Introduction
The oral cavity is an integral component of the digestive system, containing several significant anatomical components made up of various soft and hard tissues, such as teeth, oral mucosa, periodontal tissues, maxilla, and mandible. Additionally, the oral cavity is simultaneously populated largely by over 700 kinds of microorganisms, creating a complex ecological niche that directly impacts oral health [1]. Most researchers agree that dysbiosis of the microflora, rather than a particular species of bacteria, is the root cause of oral infectious disorders, including dental caries, periodontitis, peri-implantitis, and oral candidiasis [2]. Maintaining good oral health is critical in fending off periodontal diseases and dental caries, which can lead to more serious health problems, such as endocarditis, diabetes mellitus, and Alzheimer’s disease. Therefore, the prevention and treatment of these oral illnesses have thus received significant attention [3]. Oral diseases are pathological changes that occur in the soft and hard structures of the oral cavity and maxillofacial region [4]. The most common oral diseases include dental caries, periodontitis, pulp necrosis, oral mucositis, and jaw abnormalities [5]. It is estimated that more than 3~5 billion people worldwide experience chronic oral disorders that progress over time, starting in early infancy and continuing throughout adolescence, adulthood, and later life [6]. In many countries, oral disorders place a significant health burden on individuals, causing various degrees of pain, discomfort, disfigurement, and even death in some cases. Given the complex and diverse nature of oral health issues, there seems to be an endless need for dental biomaterials that can effectively interact with a range of tissues, from soft gum tissue to hard bone tissue. Moreover, dental biomaterials must be able to withstand the challenges posed by the oral environment, including abrupt temperature changes, pH fluctuations caused by saliva and biofilms, and the presence of various types of bacteria [7]. Consequently, there has been increasing research on developing effective treatments for oral illnesses. Bacterial infections frequently lead to oral disorders [8], and there are two main approaches for treating them. One aims to combat the infections, while the other aims to promote tissue regeneration. For repairing jawbone defects, autologous bone grafting is the most common treatment method currently used. However, autogenous bone is a limited source and cannot be reshaped to fit the defect. For periodontitis and oral mucositis, the prevention of bacterial infection is critical. Systemic administration has long been the primary treatment for the infectious diseases of the oral cavity. However, systemic administration may cause issues such as drug resistance and liver toxicity. For pulpal necrosis, regenerative root canal therapy is the mainstream treatment method, and the preferred scaffold for this therapy is an injectable biomaterial. However, there is no consensus on the most suitable injectable scaffold for endodontic treatment. Hydrogel, as a biocompatible material that can easily change shape, performs excellently in both drug delivery and tissue regeneration. Hydrogels usually possess a porous structure due to their internal network that forms many tiny pores and voids. The size and shape of these pores and voids can be controlled by regulating the conditions and preparation process during gel synthesis. By regulating the structure of the pores, it is possible to match them with surrounding tissues, promoting cell adhesion and growth [9,10]. Moreover, hydrogels exhibit excellent antimicrobial properties in oral clinical applications. Firstly, they physically isolate bacteria, preventing the invasion and spread of harmful microbes. Secondly, hydrogels act as effective drug release carriers, facilitating the delivery of various antimicrobial substances to achieve their desired effects. Recently, there has been considerable research on stimuli-responsive hydrogels. These hydrogels can respond to external physical, chemical, and biological stimuli, triggering the release of antimicrobial agents. Additionally, these hydrogels can exhibit antimicrobial properties through responsive physical and chemical properties, such as by modulating pore structure and regulating humidity. Therefore, hydrogel has become a promising material for the treatment of oral diseases.
2. Classification of Hydrogels
2.1. Natural Hydrogels
Hydrogels are polymer networks with a high water absorption capacity, formed by physical or chemical cross-linking. They can be categorized into two groups based on the source of their raw materials: natural hydrogels and synthetic hydrogels. Natural hydrogels include the collagen and gelatin extracted from animal proteins [28], the HA commonly found in animal epithelium and connective tissue [29], and alginate derived from the cytoplasm and cell wall of algae and seaweeds [30]. These biomaterials are non-toxic, highly biosafe, and biocompatible, making them suitable for various biomedical applications [31,32,33].
2.1.1. Collagen-Based Hydrogels
Collagen, the main component of the ECM in many mammalian tissues, provides support and protection to the organism and its organs due to its excellent coagulation effect. Collagen-based hydrogels have attracted significant attention from researchers, owing to their weak immunogenicity and good biocompatibility. These hydrogels have been widely applied in cartilage repair, dentistry, drug delivery, and corneal transplantation, with rapid development in recent years [34,35,36,37].
2.1.2. Hyaluronic Acid (HA) Hydrogels
HA, a nonsulfated glycosaminoglycan found in all connective tissue ECM, is one of the most commonly used natural polymers today [40,41]. Studies suggest that HA plays a crucial role in biological processes such as angiogenesis, ECM structure, inflammation, and wound healing. Moreover, HA derivatives have been successfully employed as scaffold biomaterials for chondrocyte development, bone regeneration, and skin tissue regeneration [42,43].
2.1.3. Gelatin Hydrogels
Gelatin is a hydrophilic polymer that possesses excellent sol–gel transition properties and biocompatibility, making it a versatile material in the field of hydrogels [45]. Hydrogels made from gelatin as a matrix can mimic various tissue characteristics and allow for the tailoring of hydrogel properties, such as mechanics and degradation, to suit a wide range of biomedical applications [46,47]. Xie et al. prepared a self-shrinking wound dressing using the oxidized starch of shape memory hydrogels, which showed through ex vivo experiments that shape memory was activated at conditions similar to human physiological temperature, thus achieving atraumatic mouth closure [48]. Customized hydrogels that mimic natural cells have been found to enhance endodontic treatment, while gelatin methacrylate-based hydrogels with modifiable physical and mechanical properties have been identified as an effective strategy to promote endodontic regeneration [49,50].
2.1.4. Alginate Hydrogels
Alginate, a natural polysaccharide isolated from brown algae or bacteria, finds wide application in tissue engineering [53,54]. Its intrinsic structure is similar to natural ECM, making it an excellent choice for biocompatible scaffolds. Pan et al. successfully regenerated alveolar skeletal and soft tissues using a bionic polysaccharide hydrogel/hydroxyapatite composite scaffold, thus presenting a new approach for clinical bone defect repair [55].
2.1.5. Chitosan (CS) Hydrogels
CS, a long-chain cationic polysaccharide, is a deacetylated derivative of chitin [58]. Its wide range of biological properties, including antibacterial, anti-inflammatory, anticancer, and tissue repair properties, have been amply demonstrated. Additionally, CS has excellent drug-loading capacity in the form of nanoparticles and hydrogels. The reactive groups (e.g., –OH, –NH2) on the chitosan backbone allow for the production of multiple derivatives with the same properties as the parent polymer, but with enhanced biocompatibility and non-toxicity [59,60,61].
2.2. Synthetic Hydrogels
Synthetic hydrogels are created through chemical reactions, as the name implies. Compared to natural hydrogels with poor mechanical properties, stability, and low bactericidal efficiency, synthetic polymer hydrogels have high molecular weight and stable mechanical properties. Moreover, the structural morphology of synthetic hydrogels can be tailored according to their intended functionality and degradability. Therefore, they can be designed with advantages such as low degradability, efficient gel formation, and a long service life. However, synthetic polymer hydrogels are typically not antimicrobial and require antimicrobial treatment [62]. Currently, synthetic hydrogels are usually made from synthetic polymers derived from natural resources, such as PLA, PEG, and GelMA [63].
2.2.1. Polylactic Acid (PLA)
PLA, a biodegradable synthetic polymer derived from abundant resources, is widely used in biomedical applications due to its excellent biocompatibility, low toxicity, biodegradability, ease of processing, and environmental friendliness [64,65]. PLA can be blended with other natural and synthetic polymers to create biodegradable PLA hydrogels [63].
2.2.2. Polyethylene Glycol (PEG)
PEG is a widely used synthetic polymer in biology due to its non-immunogenic, non-toxic, biodegradable, and highly hydrophilic nature. PEG-based hydrogels offer unique advantages in drug delivery and controlled release [69]. However, they do not provide optimal conditions for cell survival, adhesion, and development because they are inherently biologically inert.
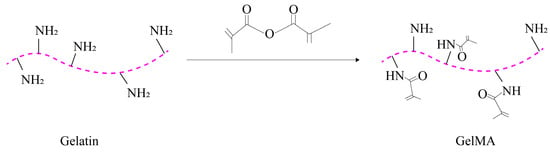
2.2.3. Gelatin Methacryloyl (GelMA)
GelMA is a photocrosslinked hydrogel that combines the properties of both natural and synthetic biomaterials. It is produced by adding methacrylate groups to gelatin [72]. The synthesis process of GelMA is illustrated in Figure 2.

Figure 2. Synthesis process of GelMA.
3. Application of Hydrogels in Oral Tissue Engineering
3.1. Hydrogels for Dental Pulp Regeneration
Dental caries, trauma, and developmental malformations can lead to the irreversible destruction of the dental pulp, which plays a crucial role in maintaining immune defense, the sensory system, and regenerating the pulp–dentin complex [74,75,76]. The dental pulp is located inside the pulp cavity of the tooth and is protected by the non-resorbing dentin hard tissue. Following conventional root canal treatment for pulpal and periapical diseases, pulpless teeth lose their structural integrity, biological defense, and sensory capacity, which can result in the susceptibility of root fractures and poor long-term outcomes [77,78]. Thus, regenerating the dentin–pulp complex is of significant importance to restore the vitality of teeth, recover the biological function of teeth, and prolong the lifecycle of pulpless teeth.
Pulp regeneration, also known as regenerative endodontic treatment, was first proposed by Murray et al. in 2007. This ideal form of regenerative therapy aims to remove the diseased or necrotic pulp tissue and replace it with healthy, vital pulp tissue [79]. In 2016, the American Association of Endodontists formally defined pulp regeneration as the use of biological procedures to replace damaged tooth structures, including the pulp–dentin complex, root, and other structures, in order to form physiological and functional pulp-like tissue and restore biological function [80].
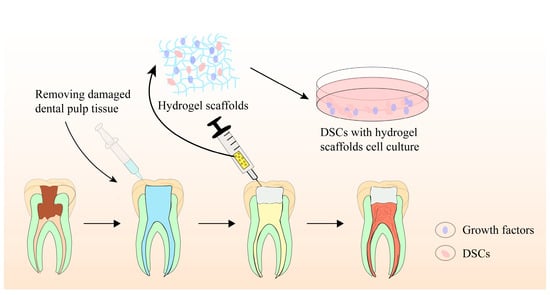
Biomaterial scaffolds play a crucial role in pulp regeneration by providing a 3D scaffold for stem cell adhesion, migration, proliferation, differentiation, and function. They not only regulate stem cell behaviors, intercellular and extracellular signaling, but also modulate the microenvironment, facilitating pulp–dentin complex regeneration. Recently, hydrogel-based scaffolds have been evaluated for tissue engineering-based pulp regeneration. These scaffolds have prominent biocompatibility, biodegradability, flexibility, elasticity, and ergonomic mechanical profiles, making them ideal candidates as cell or bioactive ingredient(s) delivery systems for promoting pulp–dentin complex regeneration. Figure 3 provides a schematic illustration of the precise pulp regeneration procedure.

Figure 3. Schematic illustration of precise pulp regeneration procedure.
Hydrogels can be classified into two categories based on the source of polymeric chain: natural and synthetic hydrogels. Natural hydrogels mimic natural peptides and possess favorable biocompatibility, but their mechanical characteristics are poor. In contrast, synthetic hydrogels exhibit distinct mechanical features and tunable physiochemical properties, but they are deficient in biocompatibility and biodegradability capacity [83].
HA is a nonsulfated glycosaminoglycan component of the ECM of soft connective tissues [86]. As an outstanding candidate scaffold for pulp regeneration, HA has excellent biocompatibility, biodegradability, non-immunogenicity, and high water content [87].
The CS hydrogel is a promising biomaterial scaffold for regenerative endodontic treatment due to its bioactivity, biocompatibility, and capacity to blend with other bioactive ingredients. Bioactivity refers to properties of materials that trigger specific biological and chemical reactions, mainly at the interface between materials and biological tissues. Chitosan, for example, has the ability to promote cell adhesion, growth, proliferation, and differentiation. Additionally, chitosan possesses antibacterial activity. Several researchers have noted that CS-based hydrogels exhibit no biological toxicity toward various cell types and promote the proliferation and differentiation of stem cells, thereby facilitating the regeneration of pulp–dentin-like tissue [90,91].
Natural hydrogels are known for their ability to mimic natural ingredients and exhibit super biocompatibility, but they carry the risk of immune reactions and poor mechanical properties. Synthetic hydrogels, on the other hand, can be engineered to possess adjustable mechanical profiles and microstructures. When equipped with bioactive molecules and cell-binding peptides, synthetic hydrogels are optimal candidates for tissue engineering applications. For example, Kuang et al. fabricated biocompatible and biodegradable PLA-based scaffolds and tested their regulatory role in dentin–pulp complex regeneration. The PLA-based scaffolds significantly promoted the proliferation and odontogenic differentiation of human dental pulp stem cells (hDPSCs) by enhancing the expression of ALP, osteocalcin, bone sialoprotein, collagen 1, and dentin sialophosphoprotein genes in in vitro experiment. Histological analysis demonstrated superior dentin-like tissue formation in vivo [96].
3.2. Hydrogels for Periodontal Tissue Regeneration
Periodontitis is a well-known oral infectious disease characterized by inflammatory and the destruction of periodontal tissue, which can lead to an accelerated loss of alveolar bone and ultimately result in teeth loss [98,99]. In 2018, a new classification of periodontal disease and peri-implant disease was established at the Joint World Symposium in Chicago. The criteria for defining periodontal health are the absence of periodontitis in intact or reduced periodontal tissue, less than 10% bleeding on probes, and periodontal pockets less than or equal to 3 mm in depth. In the new classification, periodontitis previously categorized as “chronic” or “aggressive” is now reclassified as a single “periodontitis”, with “stage” and “grade” classification. Systemic diseases and the status of affected periodontal tissue diseases have been updated. Moreover, for the first time, the classification includes peri-implant diseases and states.
Traditionally, mechanical debridement and flap surgery aiming at removing plaque have been effective in preventing the progress of inflammation and the destruction of periodontal tissue [102]. However, the reconstruction of both the structures and functions of periodontal tissue remains a great challenge and the ideal therapeutic objective of periodontal disease. Considering the physiological structure of periodontal tissues, the morphology and functional regeneration require the simultaneous or sequential repair of three components of periodontal tissue, including the periodontal ligament (PDL), which fixes the tooth, the cementum covering the root surface, and the alveolar bone supporting the tooth [103,104]. Periodontal tissue engineering has emerged as a promising technique that combines stem cells, biological scaffolds, and growth factors to promote periodontal tissue regeneration [105
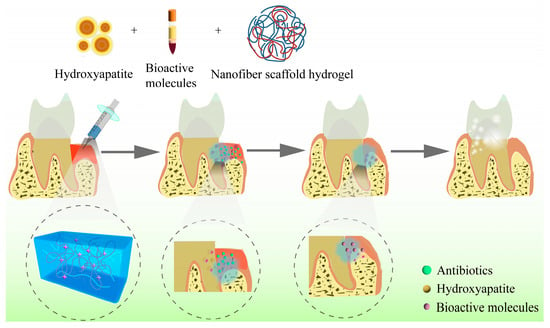
Any biomaterial scaffold, whether biological or synthetic, must be biocompatible and biodegradable when applied to tissue regeneration. Hydrogels are widely used as regenerative scaffolds in periodontal tissue engineering [110]. Due to their key characteristics of porosity, stiffness, and viscoelasticity, hydrogels can mimic the microenvironment of the ECM and facilitate the regulation of cell adhesion, proliferation, and osteogenic differentiation [111]. Studies have reported that when combined with drugs, stem cells, or growth factors, hydrogels exhibit outstanding potential in the complex and sophisticated process of periodontal tissue regeneration [112,113]. Figure 4 shows a schematic diagram of hydrogels applied in periodontal regeneration.

Figure 4. Schematic diagram of hydrogels applied in periodontal regeneration.
Collagen is the fundamental component of the ECM. It is composed of many specific cell signaling binding domains that facilitate cell adhesion, preserve cell phenotype, and guide cell growth, proliferation, and differentiation. For instance, Jung et al. reported that collagen hydrogels with different porosities can serve as a perfect scaffold for PDL repair and periodontal regeneration [114].
HA, another important component of the ECM of connective tissues and periodontal ligament matrix, has valuable potential in periodontal tissue regeneration [116,117,118,119]. Studies have shown that HA can modulate cell adhesion, migration, and differentiation by binding proteins and cell-surface receptors. When combined and modified with other ingredients, HA hydrogels exhibit excellent mechanical properties, swelling, and low degradation speed in periodontal tissue engineering.
CS is a natural cationic polymer with a chemical structure and biological properties similar to glycosaminoglycan polysaccharide. Due to its excellent biocompatibility, biodegradability, and antimicrobial activity, CS is frequently used in periodontal therapy.
3.3. Hydrogels for Mandible Regeneration
The mandible is a crucial component of the human face, as it plays a vital role in mastication, pronunciation, and speaking, while also contributing to facial contour and shape [130]. However, loss of bone tissue in the oral cavity can occur due to various reasons, such as traumas, tumors, infections, functional atrophy, congenital disorders, and periodontitis, leading to different degrees of impact on the patient’s facial appearance and oral function [131,132,133,134]. Certain drugs, such as isophosphonates, can also lead to jaw osteonecrosis, known as drug-related osteonecrosis of the jaw (MRONJ), as they are anti-angiogenic or anti-osteoporotic [135]. Even a short-term discontinuation of such drugs does not eliminate their effects on the jaws, and segmental resection becomes the only solution to remove necrotic bones in the late stage of MRONJ [136]. Although bones can heal themselves, repairing bone defects larger than a critical size and ununited fractures remains a challenge in clinical practice [137]. Moreover, self-repair is not feasible when the loss of the mandible exceeds 10% [138].
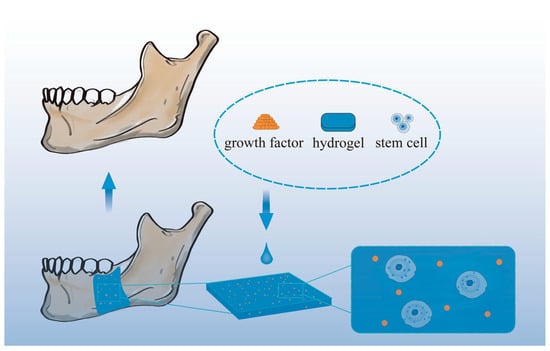
Figure 5 shows a schematic diagram of mandibular restoration using hydrogel biomaterials.

Figure 5. Schematic diagram of mandibular restoration using hydrogel biomaterials.
Hydrogel systems play a significant role in the treatment of infected jaw defects by facilitating antibiotic release. For instance, Sun et al. prepared vancomycin hydrochloride (Van)-SBA-15 using mesoporous silica (SBA-15) and encapsulated Van in a CS–sodium glycerophosphate–sodium alginate hydrogel. The hydrogel enabled the sustained release of Van and holds potential for the treatment of infected jaw defects [147]. In another study, clindamycin (CDM) was shown to penetrate human bone tissue effectively [148].
Furthermore, hydrogels have been used to mimic native bone and enhance bone regeneration. For example, Kumar et al. developed a bone tissue engineering biphasic construct loaded with bone BMP-2. They used a gelatin–HA hydrogel to bind to the osteogenic cue BMP-2, which was then loaded onto a PCL scaffold. The construct mimicked native bone and consisted of cortical bone and cancellous bone for vertical jawbone augmentation. In vitro studies showed that the cell viability of BMP-2 was maintained in the hydrogel for 21 days, and bone markers increased on the third and fourteenth days [154].
3.4. Hydrogels for Soft Tissue Healing
While dental and skeletal repair are the primary focus of craniofacial applications, the need for soft tissue regeneration is equally crucial [161]. Oral and maxillofacial soft tissues include various structures such as periodontal tissues, tongue, oral mucosa, muscles, skin. Soft tissue regeneration remains a major challenge in contemporary medicine and dentistry. Periodontitis, gingival recession, and chronic inflammation of the gums can lead to tooth loss. In the cases of long-term tooth loss, proper dental restoration and aesthetic outcomes heavily depend on critical soft tissues [162]. After dental implantation, the bacterial environment in the oral cavity can cause inflammation around the implant. Enhanced sealing of the soft tissues around the implant can improve the success of the implant [163].
Soft tissue engineering has emerged as a new approach to repair damaged or diseased soft tissues and organs [167]. Hydrogels, which are ideal biomaterials to mimic soft tissues [168], have been developed for this purpose. However, treating oral diseases can be challenging because high doses of topical medications are often required, and systemic administration is typically the main treatment option for small lesions of the oral mucosa. The moist, dynamic, and unstable environment of the oral cavity, including constant salivary flushing, and exposure to microorganisms, enzymes, and food and beverages, makes it difficult to maintain wound dressings. Furthermore, hydrogels must meet high demands for structural and mechanical stability [169].
4. Clinical Applications of Hydrogels in Oral Tissue Repair and Regeneration
There have been several clinical studies on the use of hydrogels for repairing oral tissues, and information about these studies is listed in Table 1. Most of these studies focused on the use of hydrogels for treating oral mucositis and periodontitis, with one study investigating the treatment of oral bacterial infections and another exploring tooth loss.
Table 1. Clinical applications of hydrogels in oral tissue repair and regeneration [185].
| Status | Study Title | Conditions | Interventions |
|---|---|---|---|
| Completed | Omega-3 hydrogel and prevention of oral mucositis | Mucositis oral | Drug: topical oral Omega-3 hydrogel; drug: conventional preventive treatment |
| Not yet recruiting | Efficacy of EGF-loaded self-healing gel in treating oral mucositis | Oral mucositis | Drug: EGF-loaded hydrogel; drug: hydrogel |
| Completed | A study to evaluate efficacy of MuGard for amelioration of oral mucositis in head and neck cancer patients | Oral mucositis | Device: MuGard; device: control rinse |
| Recruiting | MucoLox formulation to mitigate mucositis symptoms in head/neck cancer | Mucositis oral head and neck cancer |
Other: MucoLox; other: sodium bicarbonate |
| Unknown | Topical chamomile in preventing chemotherapy-induced oral mucositis | Oral mucositis due to chemotherapy | Drug: chamomile topical oral gel; drug: miconazole topical gel; drug: BBC oral spray; drug: oracure gel |
| Completed | Impact of daily use of emanate tray adjunct to full mouth debridement compared to full mouth debridement alone | Wound heal mouth; wound periodontal inflammation |
Device: emanate tray |
| Completed | Nitazoxanide as a new local adjunctive to nonsurgical treatment of moderate periodontitis | Periodontitis | Procedure: scaling and root planing; drug: nitazoxanide hydrogel |
| Completed | Efficacy of proanthocyanidins in nonsurgical periodontal therapy | Periodontitis, adult | Procedure: minimally invasive nonsurgical therapy; combination product: subgingival application of collagen hydrogels with proanthocyanidins; diagnostic test: collection of saliva samples |
| Completed | Does hyaluronic acid affect periodontal treatment? | Periodontitis | Procedure: scaling and root planing; drug: hyaluronic acid gel (HA) and SRP; drug: HA mouthrinse and SRP; drug: HA mouthrinse + gel and SRP |
| Completed | Use of adhesion molecule-loaded hydrogel with minimally invasive surgical technique in treating periodontal intrabony defects | Periodontitis | Drug: RGD peptide |
| Completed | PLGA nanoparticles entrapping ciprofloxacin to treat E-Fecalis infections in endodontics | Bacterial infections oral | Device: chitosan-coated PLGA nanoparticles entrapping ciprofloxacin incorporated in smart gels; device: ciprofloxacin paste and solution |
| Unknown | Hyaluronic acid effect on xenogenic bone healing | Bone resorption tooth loss |
Procedure: ridge preservation: tooth extraction and immediate bone grafting in the socket |
This entry is adapted from the peer-reviewed paper 10.3390/molecules28093946
This entry is offline, you can click here to edit this entry!
