2. Pathophysiology
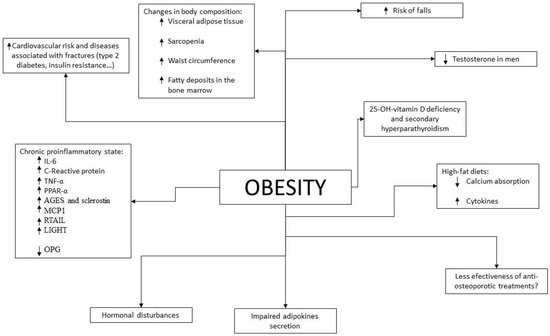
Given the results of epidemiological studies, various pathophysiological mechanisms have been investigated and described by which there could be a beneficial and/or detrimental relationship between obesity and bone fragility (Figure 1).
Figure 1. Pathophysiological mechanisms that relate obesity to bone health. Arrows indicate increase or decrease levels.
On the one hand, some papers have referred to the principle of Wolff’s law. According to it, bone adapts in response to the stress to which it is subjected. In the case of obesity, the mechanical overload produced would result in bone deformation that would trigger a cascade of transduction signals that would stimulate the maintenance of bone mass through osteoblastic activity and the Wnt/β-catenin signaling pathway [
56]. However, this would increase the quantity but not the bone quality or bone strength, and this could explain why patients with obesity fracture with higher BMD measurements than non-obese patients [
28,
57]. Furthermore, if DXA results are interpreted as a function of weight, BMD results could be considered inappropriately low [
58]. It is also postulated that the bone’s ability to adapt to mechanical overload is limited and is not maintained beyond a certain weight gain [
57].
In relation to purely mechanical mechanisms, there also falls play an important role in the pathophysiology of fractures in patients with obesity and consequently reduced mobility. These were more frequent in women with obesity in the GLOW study and resulted in greater comorbidity [
29]. In addition, falls are different from those occurring in patients without obesity, as they tend to be backward or sideways, which would explain the lower risk of wrist fracture and the higher risk of humerus fracture shown in epidemiological studies [
57]. The presence of a greater amount of subcutaneous adipose tissue in the hips in gynoid-type obesities could serve as a “cushion” in falls and explain the lower risk of hip fracture shown in epidemiological studies focusing on postmenopausal women [
58].
Another mechanism by which excess weight could increase BMD would be through the production of sex steroids by adipocytes, with widely known antiresorptive and anabolic effects. Regarding bone formation, estrogen increases osteogenic differentiation of mesenchymal stem cells (MSCs) and osteoblast maturation. With respect to bone resorption, estrogen inhibits osteoclast formation and induces osteoclast apoptosis [
59]. Postmenopausal women with obesity and increased aromatization of androgens to estrogens in subcutaneous adipose tissue would therefore have higher levels of circulating estrogens with a positive effect on bone mass and mineralization [
60]. However, it should be considered that this aromatization only occurs in subcutaneous adipose tissue, so that in obesity with a higher content of visceral adipose tissue (as occurs especially in older subjects due to the redistribution of fatty tissue related to aging) this beneficial effect is not so striking.
The same would occur in men due to the action of testosterone, which has also been associated with higher body weight [
61]. However, obese men tend to have lower testosterone levels, which has also been shown to be a risk factor for falls in older men [
62]. Obese men also tend to have lower levels of sex hormone binding globulin (SHBG), which leads to increased levels of free sex steroids. In fact, elevated levels of SHBG have been associated with lower BMD [
63] and an increased risk of fractures [
64].
Among the pathophysiological mechanisms linking obesity with increased fracture risk is 25-hydroxyvitamin D deficiency and consequent secondary hyperparathyroidism, which occur more frequently in patients with obesity. As is widely known, this has been associated with an increased risk of osteoporosis and fractures [
65]. Hyperparathyroidism is also associated with increased BMD loss in cortical bone, and this may partially explain why patients with obesity have more fractures in bones such as the humerus or ankle [
66]. In addition, diets rich in fat, which are sometimes part of the cause of obesity, interfere with intestinal absorption of calcium [
67].
At the molecular level, several pathophysiological pathways linking obesity with an increased risk of osteoporosis have also been found. Just as the chronic proinflammatory state secondary to obesity increases cardiovascular risk, it could also increase the risk of osteoporosis and fractures by altering the mechanisms of bone formation and resorption. In fact, the proinflammatory state that occurs in other diseases such as Crohn’s disease or rheumatoid arthritis has already been widely shown to increase the prevalence of osteoporosis in those patients who present with them [
68]. This pro-inflammatory state also increases the risk of insulin resistance, type 2 diabetes and arteriosclerosis, thus increasing the risk of osteoporosis and fractures as these are pathologies that also deteriorate bone quality [
69].
Adipose tissue is currently considered as an endocrine organ, since it serves as a substrate for the synthesis of sex hormones and secretes adipokines and cytokines which, among other functions, play a role in bone metabolism [
50,
70,
71]. Among the adipokines, leptin and adiponectin stand out. Their relationship with BMD has been widely studied with some controversy and discrepancy in the results obtained.
As for adiponectin, both osteoblasts and osteoclasts have receptors for it [
72,
73], and this seems to stimulate the RANKL receptor, inhibiting the production of osteoprotegerin in osteoblasts and indirectly increasing osteoclastogenesis [
74]. This is why a negative relationship between adiponectin levels and BMD has been found even after adjusting these results for total fat mass for very different groups of age and BMI [
75,
76,
77,
78]. However, this relationship in most studies is not confirmed when only premenopausal women are analyzed [
79], so it is believed that the levels of sex hormones could be a confounding factor which should be studied. On the other hand, the adiponectin effects could also be mediated by its influence on insulin levels. People with obesity have decreased levels of adiponectin compared to normal weight people, especially in those with type 2 diabetes, insulin resistance and central obesity, and it is postulated that this could be a mechanism by which obesity could be a protective factor for osteoporosis and fractures [
80].
In contrast, leptin, which is increased in patients with obesity, has receptors on osteoblasts and appears to directly stimulate osteoblast cell differentiation and inhibit osteoclast cell differentiation [
81]. This could be mediated by its inhibition of the activated NF-kB ligand receptor and a consequent increased expression of osteoprotegerin [
82]. However, it also activates the sympathetic nervous system at the hypothalamic level, which would inhibit bone formation [
83]. Currently, the relationship between leptin levels and BMD in humans shows contradictory results and it is not clear whether lectin levels ultimately have a beneficial, detrimental or neutral role in bone tissue or whether their effect is a reflection of the percentage of total fat mass [
71,
78,
81,
84]. In one study, a positive relationship between leptin levels and BMD was found to be greater in menopausal women with obesity than in the rest of the studied patients [
85], which could be due to a state of resistance to leptin in the central nervous system in patients with obesity [
86].
Moreover, cytokines produced in adipose tissue play a crucial role in the relationship between obesity, osteoporosis and fractures. Patients with obesity produce higher levels of cytokines such as IL-6, C-reactive protein and TNF-α than normoweight individuals [
87,
88]. Osteoblasts and adipocytes derive from common precursor stem cells [
89]. The differentiation of these cells into adipocyte or osteoblast depends on the activation of a series of cytokines (PPAR-α and CEBP-α, β and Δ in the case of adipocyte and RUNX2, BMP2 and TGF-β among others in the case of osteoblast) [
89]. This could even be reversed and the adipocyte returned to the precursor cell state to finally differentiate into osteoblast [
90]. It is therefore evident that there is a strong relationship between adipose and bone tissue, which depends on a cytokine microenvironment that can be altered in proinflammatory states. In patients with obesity, PPAR-α levels are increased in adipose tissue [
91], and in animal models this has also been related to fat distribution [
92].
In addition to influencing stem cell differentiation, the proinflammatory state that occurs in obesity increases the levels of cytokines that stimulate osteoclast formation and activity by affecting the RANKL/RANK/OPG pathway, such as TNF-α and IL-6 [
93,
94]. In particular, TNF-α has a direct and indirect pro-osteoclastogenic effect, as it promotes RANKL expression in bone marrow stromal cells [
95]. Obesity has also been associated with increased secretion of RANKL by osteoblasts [
96]. As for osteoblastogenesis, it is inhibited by proinflammatory cytokines and other substances are increased in obese patients such as advanced glycosylation products (AGEs) and sclerostin [
97,
98].
Another cytokine increased in patients with obesity and related to bone tissue is Monocyte Chemotactic Protein-1 (MCP1). This is expressed by various normal cells, such as fibroblasts [
99], smooth muscle cells [
100], mesotelial cells [
101], adipocytes [
99], chondrocytes [
102] and osteoblasts [
103] among others. The expression of this cytokine is increased in tumor cells [
104] and may also be downregulated when receiving corticosteroid treatment and with the increase of nitric oxide and other cytokines such as IL-13 [
105,
106,
107]. The number of MCP1 receptors as well as their levels are increased in the visceral and subcutaneous adipose tissue of obese patients when compared to patients without obesity [
108]. This is relevant since MCP1 has a pro-angiogenic action and contributes to adipose tissue expansion [
109]. In addition, it interacts with the CCR2 receptor present in monocytes and macrophages and this stimulates osteoclastogenesis through the JAK/STST and Ras/MPAK pathways [
110].
On the other hand, TRAIL is a cytokine belonging to the TNF superfamily. In undifferentiated osteoblasts it induces a pro-apoptotic signal [
111] and directly induces osteoclastogenesis in the absence of RANKL, while in its presence it has an inhibitory action [
112]. As for adipose tissue, it determines an inflammatory state [
113] and induces the proliferation of preadipocytes [
114], forming part of the pathogenesis of obesity and other metabolic diseases [
115].
Osteoprotegerin (OPG) is a soluble TRAIL with anti-inflammatory and anti-apoptotic effects [
116]. Its levels are reduced in states of obesity [
117,
118], insulin resistance [
117] and some of its complications such as non-alcoholic fatty liver disease [
119], although this has not been confirmed in all studies [
120].
The receptor LIGHT (a cellular ligand for herpes virus entry mediator and lymphotoxin receptor) is expressed by T-lymphocytes and it also belongs to the TNF superfamily. It is increased in patients with obesity and has a pro-osteoclastogenic effect. It has been shown that an elevation of its levels is related to osteoporosis [
121,
122].
The production of proinflammatory cytokines is higher in abdominal fat, while adiponectin secretion and aromatase activity is lower than in subcutaneous adipose tissue [
123,
124]. In addition, increased levels of these cytokines decrease adiponectin production. Since adiponectin could have a beneficial effect on BMD, this decrease in adiponectin levels would be detrimental [
125].
Other markers of inflammation such as C-reactive protein are also increased in patients with obesity (especially in those with abdominal obesity) [
67]. This has been associated with decreased levels of bone remodeling markers as well as lower BMD.
Finally, the role of some hormones in bone metabolism is also interesting, especially insulin. Its levels are usually elevated in patients with obesity due to a state of insulin resistance. Insulin is an anabolic hormone that contributes to bone formation by directly stimulating osteoblasts [
15]. It also reduces hepatic production of SHBG, increasing the bioavailability of estrogens and androgens [
13]. However, in states of insulin resistance, it has a direct effect on osteoclastic cells by reducing the carboxylation of osteocalcin, essential for bone mineralization. In addition, it increases the production of RANKL, increasing bone resorption [
126,
127]. Thus, insulin resistance negatively affects bone tissue [
15,
128]. However, it is controversial whether this is a direct effect or a reflection of the effects of other factors usually associated with insulin-resistant states [
129].
The role of ghrelin, a hormone increased in patients with obesity, is also still very controversial in bone metabolism and requires further study. Although in vitro a protective role on bone tissue seems to have been demonstrated [
130], human studies have shown an association only with trabecular BMD [
131]. In studies performed in bariatric surgery, the reduction of ghrelin after this procedure was associated with a greater loss of BMD [
132].
3. Changes in Body Composition during Aging
Aging produces various changes in body composition independently of changes in weight.
In terms of muscle mass, between the ages of 24 and 50 years, 10% of muscle mass is lost, to which is added a 30% loss between the ages of 50 and 80 years, with a 1% annual decrease in the fifth decade of life [
133]. This can lead to a state of sarcopenia, which is a state of decreased muscle mass and strength associated with functional limitations that may increase the risk of falls [
134]. The prevalence of sarcopenia is estimated to be 5–13% in patients aged 60–70 years, increasing to 50% in patients aged 80 years or older and being more prevalent in patients with metabolic and chronic diseases [
135]. One of the main characteristics of sarcopenia in the older subjects is fatty infiltration of muscle [
136,
137], which has been associated with an increased risk of fractures [
138].
Obesity, due to the related chronic proinflammatory state, may contribute to a greater development of sarcopenia than that produced by aging itself [
139]. The presence of obesity in patients with sarcopenia is referred to as sarcopenic obesity, which has been associated with an increased risk of morbidity and mortality [
140]. Given the aging population and the increasing prevalence of obesity, this combination is becoming increasingly prevalent leading to a public health problem, especially given the resulting increase in cardiovascular risk [
141]. It is estimated that the most important cause of fatty deposits in skeletal muscle is due to energy intake exceeding energy expenditure, resulting in energy storage in the form of adipose tissue. In people with obesity this is increased, since they have enlarged adipocytes in the subcutaneous tissue and an overload of lipid deposits that cause this excess fat to accumulate in other tissues such as muscle, following the “overflow hypothesis” [
142]. In addition to this lipid overload, adipocytes in people with obesity have a lower capacity for lipid accumulation than adipocytes in people without obesity. This fact is due to the proinflammatory state of obesity, since the increased levels of IL-6 and TNF-α reduce the expression of PPAR-γ-2 and C/EBPα, which play an important role in the correct differentiation of preadipocytes into adipocytes [
143].
On the other hand, the distribution of fat tissue itself also changes with aging, increasing visceral adipose tissue and decreasing subcutaneous adipose tissue, which goes on to infiltrate other organs such as muscle [
144]. In particular, fatty infiltration of the bone marrow is relevant, which has been related to lower bone quality [
145]. As we age, the capacity of preadipocytes to replicate, differentiate and resist apoptosis decreases due to the increase in inflammation parameters. This phenomenon is known as inflammaging [
146]. As is mentioned, this redistribution is accentuated in patients with obesity also due to alteration of the regulatory mechanisms of inflammation. This change, especially due to the increase in visceral fat, increases cardiovascular risk in older subjects, with greater relevance in patients who are also obese. As previously described, the increase in visceral adipose tissue has also been associated with a decrease in BMD and an increased risk of fractures.
Finally, it has been shown that older subjects may be particularly susceptible to the deleterious effects of obesity, since the correlation between BMI and frailty is U-shaped in these subjects, presenting the obese older subjects less aerobic capacity, less muscle strength, less physical performance and worse functionality [
147].
In summary, aging produces changes in body composition (redistribution of adipose tissue with a decrease in subcutaneous fat and an increase in visceral, intramuscular—with the consequent sarcopenia—and bone marrow deposits) that are associated with greater bone fragility and an increased risk of falls and fractures. This redistribution is similar to that produced in obesity and therefore its deleterious effects are increased in older subjects and obese patients.
4. Difficulties in the Diagnosis of Osteoporosis and Prediction of Fracture Risk in Patients with Obesity
As previously discussed, obese patients show higher BMD compared to patients without obesity on DXA. However, higher BMI and greater soft tissue thickness could alter this measurement [
148]. In addition, it seems that BMD assessment by DXA may provide inappropriate values if not interpreted in relation to weight.
As for other tests less widely used in daily clinical practice, such as high-resolution peripheral quantitative computed tomography (HRpQCT), a greater BMD has also been shown in patients with obesity, as well as greater cortical and trabecular BMD and a greater number of trabeculae in the distal radius and distal tibia, where they also present greater bone strength [
149,
150]. However, the bone size in the tibia and radius measured by this technique is not increased with respect to patients with normal weight, unlike the hip area [
149,
151]. This is in contradiction with the theory that mechanical overload in patients with obesity would contribute to increased bone formation. This technique also allows the calculation of the amount of adipose tissue in the bone marrow, which is usually increased in patients with obesity and in the older subjects and which has been related to bone microstructural deterioration and the presence of non-vertebral fractures [
152]. Like DXA, the accuracy of this test is also influenced by the thickness of the soft tissue [
153].
In patients with type 2 diabetes, a cortical strength deficit has been observed by HRpQCT, due to reduced cortical thickness and volume with increased cortical porosity in patients with microvascular complications [
154]. This has also been found to be increased in patients with type 2 diabetes with previous fractures, so it seems that these changes would contribute to an increased risk of fractures in these patients [
155].
As for bone remodeling markers, these are found to be decreased in patients with obesity when compared to patients with normal weight, this difference being greater in bone resorption markers than in bone formation markers [
156]. This reduction has also been demonstrated in patients with type 2 diabetes, independently of glucose levels [
157], which is in agreement with the results of histomorphometric studies in which signs compatible with low bone remodeling are observed [
158].
Regarding fracture risk, tools such as FRAX can underestimate it in these patients. As is known, given the description of the increase in fractures in relation to BMIs below normal, this is a parameter that is considered in this algorithm. However, given the results of older epidemiological studies previously discussed, obesity is not included as a risk factor for fractures in this tool.
There are studies that have evaluated the sensitivity of FRAX in this group of patients. In 2013, Premaor et al. [
159] compared obese postmenopausal women with non-obese women, observing that the probability calculated by FRAX for fracture at 10 years was significantly lower in the first group (7.1% vs. 10.9% in hip fracture and 18.2% vs. 23.3% in major osteoporotic fracture respectively), even if BMI was not included in the calculation (5.8% vs. 11.4% in hip fracture and 17.6% vs. 23.6% in major osteoporotic fracture). Despite this, when calculating the ROC curve, the area under the curve was similar in both groups with and without the inclusion of BMI in the calculation. It therefore suggests that the cut-off values at which to intervene may be too high for patients with obesity and lower reference values should be considered for initiating treatment. Moreover, the percentages of predicted and subsequently observed fractures were similar between groups. Another study conducted in 2014 [
160] also showed that the ability of FRAX to predict fractures did not vary with body composition.
However, the FRAX tool has two important limitations in patients with obesity: the first is that it does not predict fractures that are more frequent in this group of patients, such as ankle fractures; the second is that in patients with type 2 diabetes, increased waist circumference and/or insulin resistance it has been shown to underestimate the risk of fracture [
161]. Considering the current prevalence of obesity in older subjects, more studies are needed in the coming years to clarify this issue because of its implications.
It should be noted that, as mentioned above, patients with obesity who undergo an osteoporosis study should also be asked to have their HbA1c level measured, since diabetes influences the interpretation of the tests.
5. Prevention of Osteoporosis and Fractures in Older Subjects with Obesity
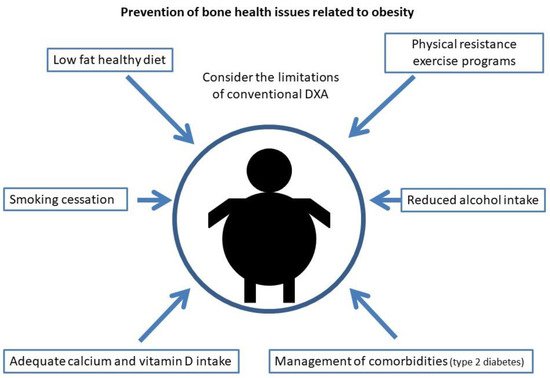
For the prevention of osteoporosis and fractures in patients with obesity, special emphasis should be placed on lifestyle measures. As in the general population, smoking and alcohol intake cessation should be advised, as well as physical exercise and a healthy diet.
Weight loss has been associated with a 1–4% loss of bone mass in the hip and trabecular bones [
162,
163,
164,
165], especially in older subjects [
166,
167]. When it occurs involuntarily, it has been associated with an increase in hip and upper limb fractures [
168], but this may be due to the loss of muscle mass that occurs when weight is lost involuntarily rather than the weight reduction itself. Studies that have evaluated intentional weight loss have shown increases in lower leg risk but a decrease in hip, pelvic and spine fractures [
168]. That distribution of fractures is similar to that occurring in patients with obesity, who are the most likely to undertake an intentional weight loss program, so these results could be biased. Furthermore, recent studies have shown that when weight loss is moderate, BMD is not reduced and bone geometry is not altered [
169]. Compared to this moderate weight loss, intense caloric restriction in a randomized clinical trial resulted in a greater loss of BMD in the hip in postmenopausal women, but not in the lumbar spine [
170]. In this same group of patients, a study showed that when BMD is lost after weight loss, it does not recover if the lost weight is regained [
171].
Given the relevance of sarcopenia in obese older patients with respect to the risk of osteoporosis and fractures, multiple studies have evaluated the role of physical exercise in these weight loss programs. In older obese patients undergoing a weight loss program, physical exercise has been shown to reduce frailty and decrease BMD and sarcopenia [
147,
172,
173] with both resistance exercise programs [
173] and aerobic combined with resistance exercise programs [
172]. On the other hand, dairy intake during weight loss has been associated with higher osteocalcin levels and increased BMD in the lumbar spine when compared to low dairy intake [
174]. In summary, in older patients with obesity, moderate weight loss should be advised in a program that includes adequate dairy intake and resistance exercise.
Regarding diet, it has been described, in experimental models, that hypercaloric and obesogenic diets are related to an increased risk of fracture by direct and indirect mechanisms [
175,
176]. High-fat diets are a risk factor for osteoporosis. In mice subjected to this type of diet, T lymphocytes isolated from the spleen and bone marrow showed increased expression of RANKL, and these mice had decreased BMD [
177], as well as increased levels of cytokines such as IL-6 and TNF-α. It has also been shown in animal models that this type of diet affects bone remodeling, triggering a loss of trabecular bone mass and also reduces calcium absorption [
178]. On the other hand, a high-fat and high-sucrose diet has been shown to affect the cortical bone in mice and rats, especially when maintained over the long term [
179,
180,
181]. In humans, data regarding the effect of a high-fat diet on the risk of osteoporosis and fracture are scarce and contradictory [
182]. However, some prospective and cross-sectional studies have shown a protective effect with protein intake [
182].
It is also worth noting the importance in these patients of an adequate intake of calcium and vitamin D, since as indicated above, high-fat diets tend to decrease calcium absorption and these patients have a high prevalence of vitamin D deficiency. As in the general population, it is recommended to obtain an optimal calcium and vitamin D intake through diet and not with supplementation if possible, especially with regard to calcium supplements that could increase arteriosclerosis [
183]. As for vitamin D, given its accumulation in adipose tissue, higher doses are usually required than in the general population.
Current measures to reduce osteoporosis and fracture risk in obesity are shown in Figure 2.
Figure 2. Current measures for the prevention of bone health issues related to obesity.