Flooding is an important strategy for weed control in paddy rice fields. However, terrestrial weeds had evolved mechanisms of tolerance to flooding, resulting in new ‘snorkelling’ ecotypes. Several weeds, mainly weedy rice, have evolved submersion tolerance mechanisms, which could be called snorkelling strategy, which are strategies that guarantee its survival and perpetuation in flooded environments. Current advances in biotechnology present the possibility of using molecular tools to understand flooding tolerance and manipulate DNA and RNA for the development of modern snorkelling weed control methods
- rice
- Echinochloa
- weedy rice
- SUB1
- SK1
- Flooding tolerance
- Hypoxia
- Rice Diversity
1. Introduction
Flooding is the most efficient cultural method for controlling flood-sensitive weeds soon after rice sowing. Water management characterizes the different rice establishment systems, such as dry-seeding, water-seeding or transplanting [1]. These systems were developed to provide water for the rice crop and to control weeds. Therefore, although rarely acknowledged, water is a natural herbicide used in flooded crop agriculture. However, since rice domestication, weeds had co-evolved with rice, and flood-tolerant weeds became one of the main problems in rice production. Transplanting and water-seeding systems are more efficient for weed control than dry-seeding, especially for weedy rice, which is one of the most important constraints of rice production worldwide. However, weedy rice is also adapting to flooding [2][3], perhaps more than the other rice weeds, and this presents a serious challenge for the utilization of water management strategies to establish rice. The changes in metabolism and morphological growth processes of weedy rice that escape flooding suggests evolution for tolerance to this abiotic stress [4].
2. Mechanisms of Tolerance to Flooding in Rice and Weedy Species
2.1. Tolerance to Flooding during Germination
Seed germination is driven by a complex regulatory system. The energy demand during germination is high; numerous exogenous and endogenous factors affect this process, mainly associated with oxygen availability. Germinating seeds in normal oxygen conditions (O2 ≥21%) derive energy via the electron transport chain and oxidative phosphorylation using O2 as final acceptor of electrons. Under anaerobic condition, the electron transport chain and oxidative phosphorylation are inhibited by lack of O2; the plant then turns on glycolysis to generate energy. Glycolysis is an inefficient process of generating ATP; thus, anoxia prevents germination of most species. To obtain sufficient energy for normal physiological function in flooded conditions, more carbohydrates are mobilized toward glycolysis; anaerobic respiration, or fermentation, is activated. The evolved ability to maintain metabolic processes under low O2 allows the germination and emergence of some species in flooded conditions. Examples of these species are Echinochloa spp., Arundinella anomala, Sagittaria montevidensis, S. trifolia, and weedy rice, which are major weeds of rice [2][5][6].
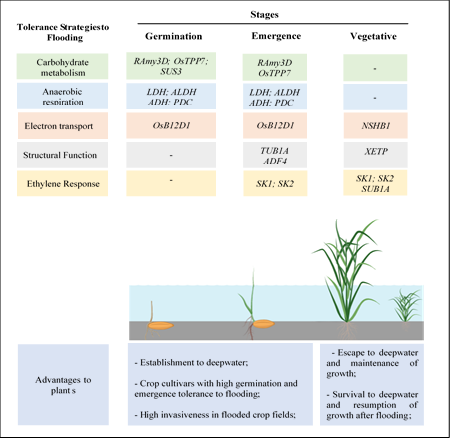
The evolved flood-tolerant ecotype of C. rotundus stores more non-structural carbohydrates, which serve as a substrate for anaerobic metabolism, compared to the normal flood-sensitive ecotype [7]. Tolerant ecotypes have larger tubers with about twice more non-structural carbohydrates than susceptible plants [7]. However, just producing more carbohydrates does not guarantee an increase in anaerobic metabolism. The plant also has to be able to convert these carbohydrates into glucose, which is the form of sugar that is transported and used in glycolysis. Therefore, adequacy in the complex network of metabolic processes is required for the evolution of tolerance to submersion. Low oxygen triggers the formation of ethylene, which induces the expression of genes related to carbohydrate conversion and maintenance of anaerobic respiration. Rice seeds that germinate well under low oxygen supply have higher expression of RAmy3D than in rice varieties intolerant to hypoxia [8]. Overexpression of RAmy3D gene in the coleoptile and endosperm of transgenic rice genotypes increased the germination capacity under anoxic conditions [9]. RAmy3D codes for alpha-amylase, which is involved in the catabolism of carbohydrates during germination. Concomitantly, the sucrose synthase (SUS3) gene promotes the production of soluble sugars when oxygen is deficient [10]; thus, providing the substrate for ATP production under oxygen-deficient environments (Figure 1).
The ATP supply for metabolism under anoxic conditions is enabled by the maintenance of the glycolytic pathway that promotes the regeneration of NAD+. This process is enabled by the consumption of pyruvate by fermentation, avoiding the accumulation of this by-product of glycolysis and decrease of the glycolytic flux due to NAD+ limitation [11]. Lactic acid fermentation starts with the production of lactate (facilitated by lactate dehydrogenase, LDH), which acidifies the cytoplasm and activates alcoholic fermentation by the action of pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH), and producing ethanol [12]. These reactions are maintained through the oxidation of NADH in the mitochondrial matrix. In this process, NADH and oxaloacetate are reduced, and reverse Krebs cycle reaction is catalyzed by malate dehydrogenase, regenerating NAD+, thereby maintaining the production of ATP [11].
Figure 1. Scheme illustrating the main genes involved in tolerance to flooding at different plant development stages and potential advantages conferred to weeds. UFRGS, June 2020. RAmy3D (Rice α-amylases); SUS3 (sucrose synthase 3); OsTPP7 (trehalose-6-phosphate phosphatase); LDH (lactate dehydrogenase); ALDH (aldehyde dehydrogenase); ADH (alcohol dehydrogenase); PDC (pyruvate decarboxylase); OsB12D1 (named OsB12D1); NShB1 (non-symbiotic hemoglobins-B); TUB1A (tubulin a-1chain); ADF4 (actin factor 4); XETP (xyloglucan endotransglicosylase); SK1 (Snorkel1) SK2 (Snorkel2); SUB1A (Submergence-1).
2.2. Tolerance to Flooding during Seedling Emergence
When germinating under flood, the rice coleoptile has to elongate to reach the water surface to perform aerobic oxidative phosphorylation. Therefore, the emergence requirements have to be supplied quickly, before the energy reserves in the seed or vegetative propagules are exhausted [4]. Coleoptile elongation, and the speed at which it occurs, constitutes one of the ‘snorkeling’ strategies for flooding tolerance. Seedling emergence in flooded conditions is facilitated by ethylene-dependent responses [10]. In one study under flooded soil, ethylene was not detected in seeds of rice cultivars IR42 and FR13A not adapted to flooding at germination, but was abundant after germination in seedlings of the flood-tolerant genotype KHO [12]. The delay in ethylene production suggests that the source of ethylene is probably the embryos that grow under oxygen deficiency, being more related to post-germination growth rather than germination per se [12].
Ethylene acts as a stimulus for the mobilization of seed reserves after germination based on the activation of alpha-amylase enzymes [10][13]. Similar to what occurs in germination, high expression of the Ramy3D gene is observed in the coleoptile and radicle of rice seedlings under anoxia (Figure 1). The expression of this gene was up to 592 times higher in the tolerant than in the susceptible genotype of rice at 8 d after seed imbibition [14]. Also associated with anoxia-tolerance in rice is the loci OssTPP7 (Gene OsTPP7), which encodes the enzyme trehalose-6-phosphate, responsible for increasing the conversion of starch to glucose [15]. This gene was identified in the flooding-tolerant cultivated rice Khao Hlan On, but was absent in the susceptible cultivar IR64, implicating OsTPP7 in flood tolerance [15]. The higher availability of sugar promoted the elongation of coleoptile, resulting in emergence and high tolerance to submersion [15][16]. Thus, these mechanisms enable seedling root and shoot growth of tolerant rice genotypes in flooded conditions. Tolerance to flooding during emergence of Echinochloa spp. Was linked to increased activity of LDH, ALDH, ADH, and PDC enzymes [17][10]. The growth of roots and shoots of flood-tolerant E. crus-galli was not affected by 100 mm depth of flood for 7 d after sowing [17].
Increased structural proteins and key enzymes have been related to continued coleoptile growth of rice seedlings under anoxia and may be the same factors involved in weed adaptation to flooding during emergence. The action of the tubulin a-1 chain (TUBA1) and actin factor 4 (ADF4) genes on the rapid elongation of coleoptile under anaerobic conditions has been proposed because both are upregulated under anoxia during rice emergence [18]. Tubulins enable cell division and elongation, as well as ADF4, is involved in the regulation of actin assembly [18]. Thus, increased expression of both genes in plants correlates with the superior ability to tolerate flooding during emergence (Figure 1). These findings indicate that there are two complex mechanisms of flooding tolerance during seedling emergence: (i) Increased mobilization of seed reserves and ii) the ability to maintain root and shoot growth during seedling emergence under flood.
2.3 Tolerance to Flooding during the Vegetative Stage
Flooding after the initial plant establishment elicits responses that differ across plant species and dependent on the speed and duration of flooding occurrence [5][10]. During initial growth, flooding stress-tolerant genotypes of rice can react by activating adaptation mechanisms to escape, or to withstand, flooding until the stress is alleviated by the formation of aerenchyma [19]. Although tolerance to flooding in rice is associated with aerenchyma, this mechanism of tolerance to flooding after establishment is also reported in other species, such as Echinochloa spp., Sagittaria montevidensis; S. trifolia, Cyperus spp., and Rumex palustris [4], or in flood-tolerant leguminous weeds of rice, such as Sesbania herbacea and Aeschynomene virginica. The presence of aerenchyma in flood-tolerant plants is also a snorkeling strategy. Aerenchyma is a plant tissue with a high proportion of intercellular spaces. The aerenchyma facilitates the diffusion of gases between the flooded and non-flooded organs of the plant, which allows the aerobic respiration of root cells [20]. The formation of aerenchyma in plants as a response to flooding is mediated by ethylene, inducing the activity of xyloglucan endotransglycosylase (XETP—Figure 1), which induces cell wall degradation. However, the formation of aerenchyma also requires reactive oxygen species (ROS) and Ca2+ in rice [21] and wheat [19]. In rice, ethylene or its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) stimulates the formation of aerenchyma in roots [22].
The presence of aerenchyma alone does not guarantee the diffusion of O2 to the root tips, because of radial oxygen loss (ROL). The rate of long-range oxygen diffusion from aerial shoots to the submerged roots is maintained by a constitutive, or induced, an apoplastic barrier that prevents the ROL [22]. This barrier is typically composed of a suberized lamella that is formed in the exodermal/hypodermic space, near the tip of the root, and by lignified sclerenchyma/epidermal cells [10]. In this way, an efficient barrier is created that minimizes ROL and increases oxygen delivery to the root tip [19].
Genes encoding non-symbiotic hemoglobins (NSHB1) was observed in rice under flood conditions as a complementary mechanism for oxygen delivery to root tips, maintaining cell energy levels under conditions of hypoxia [23] (Figure 1). Hemoglobins react with nitric oxide (NO) produced under conditions of hypoxia, favoring an alternative route of respiration and transport of electrons in the mitochondria of plants under flood. Under hypoxia, NSHB1 reacts oxidatively with NO, producing nitrate and stimulating the consumption of NADH, thereby releasing NAD+ that can be reused in glycolysis [23]. These mechanisms, acting alone or concomitantly, certainly occur in flooding-tolerant weeds. However, the specific function in different species is not known.
Aerenchyma formation is crucial in cases of complete submersion of the plant, but aeration is more efficient when the aerial part can grow above the water level [24]. Some plant species growing in flooded environments have evolved the ability to elongate porous shoots under flooded conditions to facilitate the uptake of oxygen from the atmosphere and to transport it to the roots via the aerenchyma. This adaptation has a high energy cost and is restricted to native species in environments characterized by shallow, but prolonged flooding [25]. These flood-adapted plants possess the mechanism described as low O2 escape syndrome (LOES), which is observed in some rice varieties and Rumex palustris [26].
The submersion escape mechanism is activated by low oxygen concentration and promoted by ethylene, gibberellic acid, and auxin, which collectively promote underwater growth and stretching [27]. The modulation of the response to flooding in rice is a function of the SNORKEL (SK) locus, responsible for stem elongation under deep water [28] (Figure 1). This locus encodes two ethylene-inducible transcription factors, group VII (Ethylene Response Factor (ERF): SK1 and SK2 [10]. The expression of these genes promotes elongation of the rice internodes, enabling plants to elongate underwater at a rate of 25 cm d-1 in the flood-tolerant variety C9285 [28]. The signaling effects of SK1 and SK2 on the downstream events, which culminates in plant elongation is unclear, but act on GA biosynthesis [25]. The expression of the gene SK1 was associated with flooding tolerance in ITJ03 weedy rice genotype and was not observed in flood-sensitive rice cultivars IRGA 417 [3].
The escape response to flooding is beneficial if the plant reaches the surface of the water before the plant dies. This is a high-energy-cost strategy and could exhaust the plant's carbon reserves before emergence from deep flood [26]. Thus, some species have evolved another mechanism of flood tolerance based on low O2 quiescence syndrome (LOQS), which was identified in local rice cultivars from Asia [27]. This tolerance mechanism also involves ethylene, with the SUB1 locus encoding two to three ERF-VIIs, including SUB1A, which regulates metabolism and extends the rice survival under complete submersion to up to two weeks, as well as their recovery and regrowth after the stress ends [26]. Ethylene is accumulated up to saturation level in the plant under flooding, but the ABA and GA concentrations are constant. This tolerance mechanism is also present in the weed Rumex acetosa, exhibiting metabolic adjustments consistent with energy conservation [29]. In this way, the growth of the petiole and other energy-demanding processes are suppressed, allowing the conservation of resources until the flood recedes [25].
This entry is adapted from the peer-reviewed paper 10.3390/genes11090975
References
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed Management in Direct-Seeded Rice. Adv. Agron. 2007, 93, 153–255.
- Chauhan, B.S. Weedy Rice (Oryza sativa) II. Response of Weedy Rice to Seed Burial and Flooding Depth. Weed Sci. 2012, 60, 385–388.
- Kaspary, T.E.; Cutti, L.; Rafaeli, R.S.; Delattore, C.A.; Merotto, A. Genes Related to Flooding Tolerance during Germination and Early Growth of Weedy Rice. Weed Res. 2020, in press.
- Abdelbagi M. Ismail; David E. Johnson; Evangelina S. Ella; Georgina V. Vergara; Aurora M. Baltazar; Adaptation to flooding during emergence and seedling growth in rice and weeds, and implications for crop establishment. AoB PLANTS 2012, 2012, pls019, 10.1093/aobpla/pls019.
- Xiao Qi Ye; Jin Liu Meng; Bo Zeng; Ming Wu; Improved flooding tolerance and carbohydrate status of flood-tolerant plant Arundinella anomala at lower water temperature. PLOS ONE 2018, 13, e0192608, 10.1371/journal.pone.0192608.
- Bo Liu; Ming Jiang; Shouzheng Tong; Wenguang Zhang; Haitao Wu; Ying Liu; Xianguo Lu; Differential Flooding Impacts on Echinochloa caudata and Scirpus planiculmis: Implications for Weed Control in Wetlands. Wetlands 2016, 36, 979-984, 10.1007/s13157-016-0805-0.
- Jennifer T. Peña-Fronteras; Mizpah C. Villalobos; Aurora M. Baltazar; Florinia E. Merca; Abdelbagi M. Ismail; David E. Johnson; Adaptation to flooding in upland and lowland ecotypes of Cyperus rotundus, a troublesome sedge weed of rice: tuber morphology and carbohydrate metabolism. Annals of Botany 2008, 103, 295-302, 10.1093/aob/mcn085.
- Sheng-Kai Hsu; Chih-Wei Tung; RNA-Seq Analysis of Diverse Rice Genotypes to Identify the Genes Controlling Coleoptile Growth during Submerged Germination. Frontiers in Plant Science 2017, 8, 762, 10.3389/fpls.2017.00762.
- Chung-Shen Wu; Wei-Tin Kuo; Chia-Yu Chang; Jun-Yi Kuo; Yi-Ting Tsai; Su-May Yu; Hsi-Ten Wu; Peng-Wen Chen; The modified rice αAmy8 promoter confers high-level foreign gene expression in a novel hypoxia-inducible expression system in transgenic rice seedlings. Plant Molecular Biology 2014, 85, 147-161, 10.1007/s11103-014-0174-0.
- Takeshi Fukao; Kenong Xu; Pamela C. Ronald; Julia Bailey-Serresa; A Variable Cluster of Ethylene Response Factor–Like Genes Regulates Metabolic and Developmental Acclimation Responses to Submergence in Rice. The Plant Cell 2006, 18, 2021-2034, 10.1105/tpc.106.043000.
- Marcio Rocha; Francesco Licausi; Wagner L. Araújo; Adriano Nunes-Nesi; Ladaslav Sodek; Alisdair R. Fernie; Joost T. Van Dongen; Glycolysis and the Tricarboxylic Acid Cycle Are Linked by Alanine Aminotransferase during Hypoxia Induced by Waterlogging of Lotus japonicus. Plant Physiology 2010, 152, 1501-1513, 10.1104/pp.109.150045.
- Abdelbagi M. Ismail; Evangelina S. Ella; Georgina V. Vergara; David J. Mackill; Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Annals of Botany 2008, 103, 197-209, 10.1093/aob/mcn211.
- Berta Miro; Abdelbagi M. Ismail; Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Frontiers in Plant Science 2013, 4, 269, 10.3389/fpls.2013.00269.
- Rasika Lasanthi-Kudahettige; Leonardo Magneschi; Elena Loreti; Silvia Gonzali; Francesco Licausi; Giacomo Novi; Ottavio Beretta; Federico Vitulli; Amedeo Alpi; Pierdomenico Perata; et al. Transcript Profiling of the Anoxic Rice Coleoptile. Plant Physiology 2007, 144, 218-231, 10.1104/pp.106.093997.
- Tobias Kretzschmar; Margaret Anne F. Pelayo; Kurniawan R. Trijatmiko; Lourd Franz M. Gabunada; Rejbana Alam; Rosario Jimenez; Merlyn S. Mendioro; Inez H. Slamet-Loedin; Nese Sreenivasulu; Julia Bailey-Serres; et al. A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nature Plants 2015, 1, 15124, 10.1038/nplants.2015.124.
- Bianka Steffens; Margret Sauter; Role of Ethylene and Other Plant Hormones in Orchestrating the Responses to Low Oxygen Conditions. Lipids in Photosynthesis: Structure, Function and Genetics 2013, 21, 117-132, 10.1016/j.pbi.2016.06.005.
- Lucy P. Estioko; Berta Miro; Aurora M. Baltazar; Florinia E. Merca; Abdelbagi M. Ismail; David E. Johnson; Differences in responses to flooding by germinating seeds of two contrasting rice cultivars and two species of economically important grass weeds. AoB PLANTS 2014, 6, 1-15, 10.1093/aobpla/plu064.
- Irfan Sadiq; Francesca Fanucchi; Eleonora Paparelli; Emanuele Alpi; Angela Bachi; Amedeo Alpi; Pierdomenico Perata; Proteomic identification of differentially expressed proteins in the anoxic rice coleoptile. Journal of Plant Physiology 2011, 168, 2234-2243, 10.1016/j.jplph.2011.07.009.
- Katsuhiro Shiono; Satoshi Ogawa; So Yamazaki; Hiroko Isoda; Tatsuhito Fujimura; Mikio Nakazono; Timothy David Colmer; Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Annals of Botany 2010, 107, 89-99, 10.1093/aob/mcq221.
- Takaki Yamauchi; Kohtaro Watanabe; Aya Fukazawa; Hitoshi Mori; Fumitaka Abe; Kentaro Kawaguchi; Atsushi Oyanagi; Mikio Nakazono; Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions. Journal of Experimental Botany 2013, 65, 261-273, 10.1093/jxb/ert371.
- David E. Evans; Aerenchyma formation. New Phytologist 2003, 161, 35-49, 10.1046/j.1469-8137.2003.00907.x.
- Timothy D Colmer; M. C. H. Cox; L. A. C. J. Voesenek; Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 2006, 170, 767-778, 10.1111/j.1469-8137.2006.01725.x.
- Christos Dordas; Nonsymbiotic hemoglobins and stress tolerance in plants. Plant Science 2009, 176, 433-440, 10.1016/j.plantsci.2009.01.003.
- Max Herzog; Ole Pedersen; Partial versus complete submergence: snorkelling aids root aeration inRumex palustrisbut not inR. acetosa. Plant, Cell & Environment 2014, 37, 2381-2390, 10.1111/pce.12284.
- Rashmi Sasidharan; Laurentius A. C. J. Voesenek; Ethylene-Mediated Acclimations to Flooding Stress. Plant Physiology 2015, 169, 3-12, 10.1104/pp.15.00387.
- L. A. C. J. Voesenek; Julia Bailey-Serres; Flood adaptive traits and processes: an overview. New Phytologist 2015, 206, 57-73, 10.1111/nph.13209.
- Julia Bailey-Serres; Takeshi Fukao; Daniel J. Gibbs; Michael J. Holdsworth; Seung Cho Lee; Francesco Licausi; Pierdomenico Perata; Laurentius A.C.J. Voesenek; Joost T. Van Dongen; Making sense of low oxygen sensing. Trends in Plant Science 2012, 17, 129-138, 10.1016/j.tplants.2011.12.004.
- Yoko Hattori; Keisuke Nagai; Shizuka Furukawa; Xian-Jun Song; Ritsuko Kawano; Hitoshi Sakakibara; Jianzhong Wu; Takashi Matsumoto; Atsushi Yoshimura; Hidemi Kitano; et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 2009, 460, 1026-1030, 10.1038/nature08258.
- Hans Van Veen; Angelika Mustroph; Gregory A. Barding; Marleen Vergeer-Van Eijk; R. A. M. Welschen-Evertman; Ole Pedersen; Eric J.W. Visser; Cynthia K. Larive; Ronald Pierik; Julia Bailey-Serres; et al. Two Rumex Species from Contrasting Hydrological Niches Regulate Flooding Tolerance through Distinct Mechanisms. The Plant Cell 2013, 25, 4691-4707, 10.1105/tpc.113.119016.