Clear cell renal cell carcinoma (ccRCC) is a type of kidney cancer that arises from the cells lining the tubes of the kidney. The tumor immune microenvironment (TIME) of ccRCC is a complex interplay of various immune cells, cytokines, and signaling pathways. One of the critical features of the ccRCC TIME is the presence of infiltrating immune cells, including T cells, B cells, natural killer cells, dendritic cells, and myeloid-derived suppressor cells. The complex interplay between the immune system and the tumor in ccRCC has important implications for developing new treatment strategies. Immunotherapy, which aims to activate the immune system to recognize and eliminate tumor cells, has shown promise in the treatment of ccRCC, and several immune-based therapies have been approved for clinical use.
1. Immune Checkpoint Inhibitors
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block inhibitory molecules on T cells or their ligands on tumor cells, activating the anti-tumor immune response. The most widely studied ICIs in ccRCC are PD-1 and CTLA-4, showing efficacy in the treatment of ccRCC. PD-1 is an inhibitory receptor expressed in T cells, while PD-L1 is a ligand expressed in tumor cells and other immune cells. The binding of PD-1 to PD-L1 inhibits T cell activation and promotes immune evasion [
20]. Thus, PD-1/PD-L1 inhibitors can block this interaction, leading to the activation of the anti-tumor immune response. Several PD-1/PD-L1 inhibitors have been approved to treat advanced ccRCC, including nivolumab, pembrolizumab, and atezolizumab.
CTLA-4 is another inhibitory receptor expressed on T cells, which competes with the co-stimulatory receptor CD28 for binding to its ligands on antigen-presenting cells. CTLA-4 inhibitors block this interaction, leading to the activation of the anti-tumor immune response. Examples of this inhibitor include the approved CTLA-4 inhibitor ipilimumab, which has been approved for treating advanced ccRCC.
In a phase 3 clinical trial, treatment with the anti-PD-1 antibody nivolumab significantly improved overall survival compared to everolimus, a standard therapy for advanced ccRCC [
61]. Similarly, the combination of nivolumab and ipilimumab has improved progression-free survival and overall survival compared to sunitinib, a standard first-line therapy for advanced ccRCC [
62].
In addition, other checkpoint inhibitors, such as antibodies targeting T cell immunoglobulin (VISTA), mucin domain 3 (TIM-3), lymphocyte activation gene 3 (LAG-3), and TIGIT, are also being evaluated in clinical trials for the treatment of ccRCC [
63].
VISTA, or the V-domain immunoglobulin suppressor of T cell activation, is a checkpoint molecule expressed on immune cells and some tumor cells. It appears to play a role in suppressing T cell function, allowing tumors to evade the immune system. In addition, studies have found that VISTA expression is elevated in ccRCC tumors and that targeting VISTA with antibodies can enhance the anti-tumor immune response [
64].
TIM-3 (T cell immunoglobulin and mucin domain 3) is another checkpoint protein expressed on T cells, NK cells, and other immune cells. TIM-3 regulates immune cell activation and function and has been implicated in tumor immune evasion. Studies have found that TIM-3 expression is elevated in ccRCC tumors, and that blocking TIM-3 can enhance T cell activity and reduce tumor growth in preclinical models [
65].
Another checkpoint protein is LAG-3 (lymphocyte activation gene 3), expressed on immune cells, which regulates T cell function. Like VISTA, LAG-3 is upregulated in ccRCC tumors, and blocking it with antibodies has been shown to enhance T cell activity and reduce tumor growth in preclinical models [
66].
Lastly, TIGIT (T cell immunoglobulin and ITIM domain) is a checkpoint molecule expressed on NK and T cells. TIGIT interacts with several ligands, including CD155, expressed on some tumor cells. Studies have found that TIGIT expression is elevated in ccRCC tumors and that blocking TIGIT can enhance the anti-tumor immune response [
67].
On the other hand, using nanotechnology for medical purposes has emerged as a promising approach for treating ccRCC. However, it is still at the research level. One of the major challenges in the treatment of ccRCC is the delivery of therapeutic agents to the tumor site [
68]. Nanoparticles can overcome this challenge by selectively accumulating in tumors due to the enhanced permeability and retention effect.
Several nanomedicine-based therapies have been explored to treat ccRCC, including targeted drug delivery, gene therapy, and immunotherapy [
69].
One example of a nanomedicine-based therapy for ccRCC is liposomes, which are spherical structures comprising a lipid bilayer that can encapsulate drugs and other therapeutic agents. In one study, liposomes delivered a small interfering RNA (siRNA) targeting hypoxia-inducible factor 2α (HIF-2α), which is overexpressed in ccRCC. In addition, the liposomes could selectively deliver the siRNA to the tumor site and reduce HIF-2α expression, leading to mouse tumor regression [
70].
Another approach involves using nanoparticles as carriers for immunotherapeutic agents such as cytokines or checkpoint inhibitors. For example, one study used nanoparticles to deliver interleukin-2 (IL-2), a cytokine that stimulates the immune system to attack cancer cells. The nanoparticles increased the accumulation of IL-2 in the tumor, leading to improved antitumor activity [
71]. Another research group developed polymer proteolysis targeting chimeras as a targeted delivery system for cancer models [
72].
In addition to these approaches, ongoing efforts are underway to develop novel nanomedicine-based therapies for ccRCC. For example, researchers are exploring the use of nanorobots, nanoscale devices that can be programmed to target specific cells or tissues to treat ccRCC [
73].
Overall, nanomedicine-based approaches show great promise for the treatment of ccRCC, and ongoing research in this field is likely to lead to the development of more effective and targeted therapies in the future.
2. Targeted Therapies
Targeted therapies, which aim to inhibit specific signaling pathways that promote tumor growth and survival, have also been developed to treat ccRCC. For example, mitochondrial metabolism has been shown to play a critical role in the progression of ccRCC [
74]. In ccRCC, there is an increase in the activation of the hypoxia-inducible factor (HIF) pathway, which leads to the upregulation of glycolysis and the downregulation of mitochondrial metabolism [
75]. This shift towards glycolysis, known as the Warburg effect, is thought to provide a metabolic advantage to cancer cells by allowing them to generate energy and biomass rapidly.
However, recent studies have shown that targeting mitochondrial metabolism can disrupt the progression of ccRCC. One of the key regulators of mitochondrial metabolism is the enzyme pyruvate dehydrogenase kinase (PDK), which inhibits the activity of pyruvate dehydrogenase (PDH), a critical enzyme in the mitochondrial metabolism pathway [
76]. Inhibition of PDK leads to increased activity of PDH, which promotes mitochondrial metabolism and a decreased reliance on glycolysis.
Studies have shown that inhibition of PDK can suppress the growth of ccRCC cells in vitro and in vivo. Additionally, targeting other components of mitochondrial metabolism, such as the electron transport chain, has also been shown to have anti-tumor effects in ccRCC. These findings suggest that targeting mitochondrial metabolism may be a promising therapeutic approach for the treatment of ccRCC.
Other therapies include TKIs and inhibitors of the mammalian target of rapamycin (mTOR). While these therapies have shown efficacy in treating ccRCC, they can also have immunosuppressive effects, which may limit their effectiveness in combination with immunotherapy [
60]. However, recent studies have shown that combining targeted therapies with immune checkpoint inhibitors may improve patient outcomes (
Figure 3) [
25,
77].
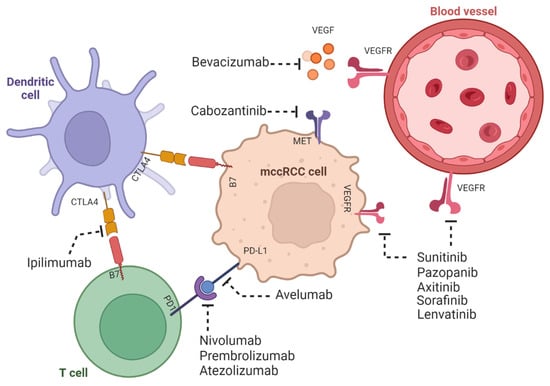
Figure 3. ccRCC tumors are both angiogenic and immunogenic. The mechanisms of the immunogenic response of mccRCC with dendritic and T cells and its effect in angiogenesis. Shown are the therapies involving tyrosine kinase and immune check inhibitors. The figure was created using
BioRender.com.
3. Combination Therapies
Combining immune checkpoint inhibitors with other immunomodulatory agents, such as cytokines, vaccines, or cellular therapies, is a promising strategy for improving the efficacy of immunotherapy in ccRCC. For example, combining the checkpoint inhibitor atezolizumab with the cytokine IL-2 has shown promising results in a phase 1b clinical trial [
78]. Similarly, combining the anti-PD-1 antibody pembrolizumab with a cancer vaccine has shown promise in phase 2 clinical trials [
79]. In addition, cellular therapies, such as chimeric antigen receptor (CAR) T cells, are also being evaluated as a potential treatment for ccRCC. However, more research is needed to determine their safety and efficacy [
80].
4. Adoptive Cell Therapy
Adoptive cell therapy (ACT) involves transferring ex vivo expanded immune cells, such as T cells or NK cells, back into the patient to enhance the anti-tumor immune response. ACT has shown promising results in treating several cancers, including melanoma and leukemia, but its efficacy in ccRCC is still under investigation [
81].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24097946