Despite the many strategies employed to slow the spread of cancer, the development of new anti-tumor drugs and the minimization of side effects have been major research hotspots in the anti-tumor field. Natural drugs are a huge treasure trove of drug development, and they have been widely used in the clinic as anti-tumor drugs. Selaginella species in the family Selaginellaceae are widely distributed worldwide, and they have been well-documented in clinical practice for the prevention and treatment of cancer. Biflavonoids are the main active ingredients in Selaginella, and they have good biological and anti-tumor activities, which warrant extensive research. The promise of biflavonoids from Selaginella (SFB) in the field of cancer therapy is being realized thanks to new research that offers insights into the multi-targeting therapeutic mechanisms and key signaling pathways. The pharmacological effects of SFB against various cancers in vitro and in vivo are reviewed in this review. In addition, the types and characteristics of biflavonoid structures are described in detail; we also provide a brief summary of the efforts to develop drug delivery systems or combinations to enhance the bioavailability of SFB monomers. In conclusion, SFB species have great potential to be developed as adjuvant or even primary therapeutic agents for cancer, with promising applications.
- Selaginella
- biflavonoids
- anticancer
1. 简介
New growths produced by the expansion of local tissue cells in response to many tumorigenic triggers are referred to as “tumors”. It is primarily a mass protrusion that occupies space, also known as a neoplasm [1,2]. In terms of morbidity and mortality, cancer continues to be a major global public health issue, coming in second place only behind cardiovascular disease [3,4]. A report on worldwide cancer statistics published in 2020 by the International Agency for Research on Cancer indicated that, in this century, cancer might surpass cardiovascular disease as the major cause of premature death in the majority of countries. By 2040, there are projected to be 28.4 million new cases of cancer worldwide, an increase of 47% from 2020 [5,6]. The pathogenic mechanisms of cancer include maintaining proliferative signals, eluding growth inhibitors, avoiding cell death, establishing replicative immortality, initiating angiogenesis, and triggering invasion and metastasis [7]. Over the last 50 years, a lot of strategies have been employed to slow the spread of cancer, namely surgery, radiotherapy, and systemic therapy [8,9]. These treatments, however, have numerous limitations and side effects, such as a high incidence of drug resistance and multidrug resistance, low efficacy of some targeted therapies, and severe adverse responses, whether administered alone or in combination [10,11]. Thus, developing novel anti-tumor medications and minimizing side effects have consistently been major areas of research in the anti-tumor field.
Due to their multi-channel and multi-target characteristics, anti-tumor compounds derived from natural products are typically used to treat advanced cancer and relieve early cancer symptoms. Herbs used in traditional medicine are the source of most natural products [12–14]. Over the past few decades, natural products have been a significant source for the development of new anti-tumor drugs [11]. Currently, more than 100 natural compounds are clinically used to treat cancers [15,16]. For example, studies have demonstrated that the active ingredient in Curcuma longa, curcumin, exerts anti-tumor effects by boosting apoptosis, inhibiting cell proliferation, obstructing tumor angiogenesis and metastasis, and inducing autophagy [17,18]. For example, paclitaxel exerts its anti-breast cancer effects by blocking mitosis (affecting B-cell lymphoma 2 (Bcl-2) phosphorylation), controlling microtubule polymerization, affecting calcium signaling, and regulating microRNA expression profiles [19]. Furthermore, significant anti-cancer efficacy is exhibited by artemisinin, which can generate reactive oxygen species in cancer cells, induce cell cycle arrest and autophagy, block cancer cell invasion and migration, and accelerate cancer cell apoptosis [20].
In recent years, a lot of literature has been reported about the classical anti-tumor signaling pathways of natural products, which are found to have the advantages of multiple targets and pathways, providing a sufficient theoretical basis for natural products as more promising antitumor drugs. For instance, the phosphoinositide 3-kinase (PI3K) signaling pathway is a crucial signaling pathway for normal cell physiological metabolism. By inhibiting this signaling pathway, tumor growth and metastasis can be prevented. Activation of PI3K can also assist in the phosphorylation of Akt and, ultimately, together play a crucial role in tumor progression [21]. For instance, Yan et al. [22] found that baicalein induced apoptosis and autophagy in breast cancer cells by inhibiting PI3K/Akt signaling pathway. In addition to the PI3K signaling pathway, the signal transducer and activator of the transcription 3 (STAT3) signaling pathway is one of the key targets for cancer therapy, which is a significant intracellular signal transduction protein and transcription factor directly related to the growth of tumors [23]. Luo et al. [24] found that Bavachin induces iron death in osteosarcoma cells via inhibition of STAT3 activity. Additionally, mitogen-activated protein kinase (MAPK) is a crucial intracellular signal transduction system that controls a variety of processes in cells, including cell growth, proliferation, differentiation, apoptosis, adhesion, and migration. These processes have an impact on drug resistance, invasion, and metastasis, as well as tumorigenesis, making it one of the potential targets of anti-tumor medications [25].
With more than 700 species recognized, the genus Selaginella of the family Selaginellaceae is extensively distributed around the world; especially, it is primarily found in tropical and subtropical areas. These species have been reported to possess many bioactivities that include anticancer, anti-inflammatory, antibacterial, antiviral, antioxidant, anti-aging, hypoglycemic, and other activities [26,27]. However, the current pharmacological studies of Selaginella plants are mainly focused on anti-tumor effects. For example, Ethyl acetate fractions from Selaginella doederleinii exhibited cytotoxicity effects for A549 cell lines, 7721 cell lines, Hela cell lines and Eca-109 cell lines, among which it has the strongest inhibitory effect on Hela cell lines with IC50 value of 37.53 μg/mL [28]. Li et al. [29] discovered that ethyl acetate extract from Selaginella doederleinii induced autophagic death and apoptosis in colorectal cancer cells through the PI3K-Akt-mTOR and AMPKα signaling pathways. Lei J et al. [30] found that the biflavonoid extracts from S. moellendorffii exhibited a noticeable negative effect on the growth rate of HCT-116 cell lines and HeLa cell lines in the range of 0 μg/mL to 500 μg/mL. Qin et al. [31] used proline-lactic acid to prepare biflavonoid extracts, which showed significant inhibitory activity against tumor A549 cells, sw1990 cells and HepG2 cells. Therefore, we comprehensively discussed biflavonoids from Selaginella, which are the main active ingredient that exerts anti-tumor effects and devoted ourselves to elucidating its anti-tumor mechanism of action and its signaling pathway in the review. As shown in Figure 1.
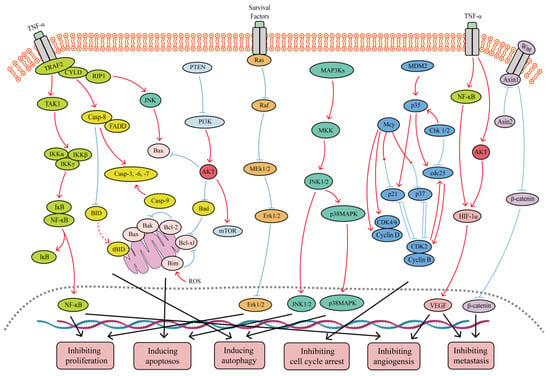
Figure 1. Major signaling pathways involved in the anticancer effects of SFB. SFB can inhibit the TNF-α/NF-κB, PI3K/Akt, Wnt/β-catenin, Ras/MEK/Erk, and PI3K/Akt/mTOR signaling pathways. In addition, SFB can activate the JNK1/2, p38 MAPK and mitochondrion-mediated caspase-dependent apoptotic signaling pathways. Red curves indicate inhibition, and blue arrows indicate activation of these processes.
In the last two decades, scientists have discovered that Selaginella plants have a wide range of active components, mostly classified as alkaloids, selaginellins and phenolic acids. Selaginellin components have been found to have potential anti-tumor activity due to their unique triple-bond structure. For example, Zhang et al. [32] isolated five selaginellin derivatives from Selaginella tamariscina, and two of the new selaginellins were structurally characterized by spectroscopic analysis. Cytotoxic activity assessment showed that the two new selaginellins exhibited moderate toxicity against human cancer cell lines (U251, HeLa, MCF-7). In addition, Thamnarak et al. [33] isolated two new ortholignans siamensinols (1–2) and seven known compounds agatharesinol (3), syringaresinol-glucoside (4), noreugenin from Selaginella siamensis (5), 8- methyleugenitol (6), melachromone (7), uncinoside A (8), and daucosterol (9). Among them, compounds 1–2 showed moderate inhibitory effects on MOLT-3 cells, while compounds 6-8 showed moderate inhibitory effects on three tumor cells (HepG2, A549 and HuCCA-1).
- Structural Characteristics of SelaginellaBiflavonoid
Biflavonoids are the primary active ingredients of Selaginella plants, exerting anti-cancer effects [26]. SFB species exert anti-tumor effects by inhibiting cancer cell proliferation, inducing cancer cell apoptosis, inhibiting tumor metastasis and angiogenesis, inducing autophagy, and impacting the tumor microenvironment. As shown in Figure 2. SFB has been shown to have anti-cancer effects on a variety of tumors, including ovarian cancer, lung cancer, prostate cancer, breast cancer and digestive system tumors.
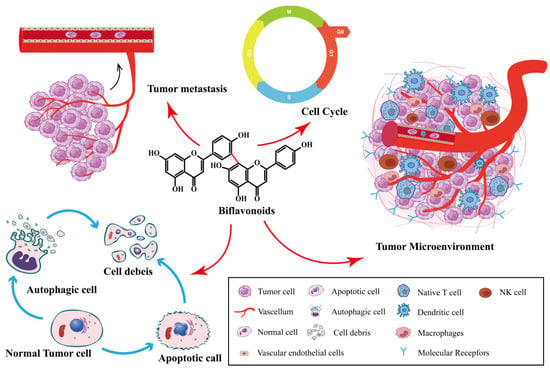
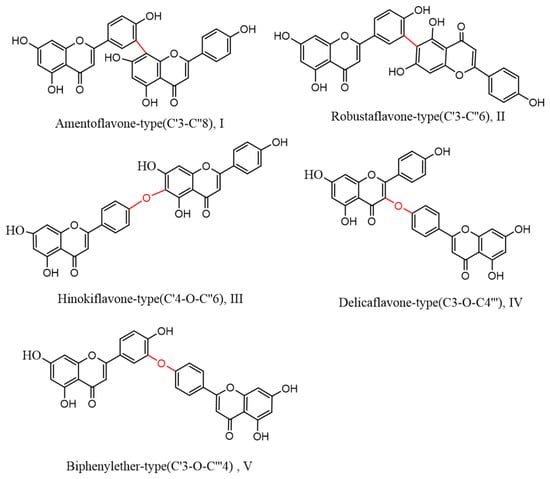
Figure 4. Nine species of bioflavonoids from Selaginella doederleinii have been recently discovered.
Biflavonoids derived from plants other than those of the genus Selaginella also have significant anti-tumor effects. According to reports, inhibiting Eg5 can cause apoptosis and mitotic arrest. Eg5 inhibitors are therefore becoming the focus of anticancer drug research. As a mitotic kinesin Eg5 inhibitor, morelloflavone from Garcinia dulcis binds to the variable site of Eg5, thereby inhibiting the ATPase activity and motor function of Eg5 [47]. Chamaejasmenin isolated from Stellera chamaejasme L. roots inhibited the proliferation of cells from eight human solid tumor cell lines (HepG2, SMMC-7721, A549, MG63, U-2 OS, and KHOS, HCT-116 and HeLa) [48]. Japoflavone D, a biflavonoid from Lonicera japonica flower buds with significant antioxidant activity, has a dual regulatory effect on the apoptosis of liver cancer cells under various oxidative circumstances [49]
This entry is adapted from the peer-reviewed paper 10.3390/ijms24097731
