You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Insects, such as wasps, ants, and bees, can live in highly structured societies characterized by a complex organization. The functioning of these societies is achieved through the coordination of several individuals who can be involved in various tasks and whose numbers are regulated to respond to the overall colony status or needs. The regulatory mechanisms of social behavior are not fully unraveled, but molecules such as brain biogenic amines likely play a pivotal role.
- dopamine
- tyramine
- serotine
- octopamine
- modulation
- regulation
- social behavior
1. Introduction
Sociality is the main trait characterizing insects like bees, wasps, ants, and termites. Thanks to their complex organization, social insects are among the most widespread and ecologically dominant animals worldwide, present in almost all environmental niches. Their success can be ascribed to the strict organization of the colonies, the reproductive strategies, the effective division of labor, and decision-making [1][2]. The most advanced type of sociality and organization is “eusociality”, which is historically grounded on the presence of three main paradigms [1]: (i) Cooperative brood care—the offspring maintenance is shared among various colony members; (ii) Overlapping generations—the coexistence of individuals with different ages or belonging to distinct generations in the same colony; (iii) Reproductive division of labor culminating in the existence of casts. Recently, eusociality has been primarily defined by the reproductive division of labor [3][4].
The social structure is maintained through a complex communication system based on chemical [5][6], acoustical [7][8][9], visual [10][11], and tactile signals [12], as well as benevolent (e.g., trophallaxis or grooming behavior) or aggressive interactions to establish dominance dynamics in the colony hierarchy. Multimodal signals [13][14] are perceived by various sensors and integrated into the central nervous system in the brain of colony members. However, social insects show high flexibility in their responses based on the overall colony status. This plasticity is supposedly achieved by combining multiple biochemical pathways through the neuromodulation of biogenic amines, neuropeptides, and hormones, but the mechanisms through which these regulatory molecules act are far from being fully disentangled [15][16][17].
Among neuroactive compounds, biogenic amines (BAs) are known to function as neurotransmitters in the synaptic gap, neuromodulators of local cell circuits, or neurohormones acting remotely in other body compartments [18][19][20][21][22]. BAs are nitrogen compounds with one (or more) amine groups originating from the decarboxylation of amino acids. An amazing diversity of BAs has been described, with some compounds acting as toxins, while only a few playing a significant physiological role, which differs between vertebrates and invertebrates [20][23][24].
The main BAs used as neurotransmitters in social insects are tyramine (TA), dopamine (DA), L-3,4-dihydroxyphenylalanine (L-DOPA), octopamine (OA), and serotonin (5-HT). L-DOPA, DA, TA, and OA are derived from the aromatic amino acid phenylalanine, converted to tyrosine by a hydroxylase (Enzyme Commission number EC: 1.14.16.1). The newly synthesized amino acid can be subsequently converted, according to physiological need, into the four different BAs. Specifically, it is converted into L-DOPA and DA by the action of Tyrosine Hydroxylase (EC: 1.14.16.2) and DOPA Decarboxylase (EC: 4.1.1.26). Otherwise, it is transformed into TA and OA by Tyrosine Decarboxylase (EC: 4.1.1.25) and Tyramine Hydroxylase (EC: 1.14.17.1), respectively (Figure 1) [25][26].
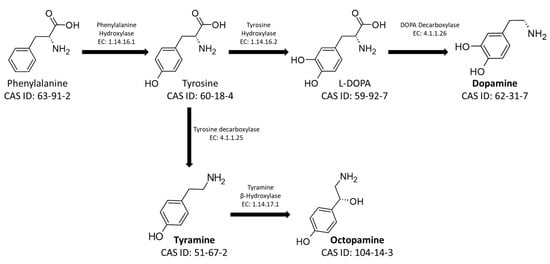
Figure 1. Metabolic pathways for the synthesis of the biogenic amines Tyramine, Octopamine, L-DOPA, and Dopamine. The figure shows the CAS-IDs of the molecules along with the EC numbers of the enzymes involved in the biosynthetic pathway.
On the other hand, the 5-HT pathway starts from the aromatic amino acid tryptophan, which is hydroxylated by Tryptophan Hydroxylase (EC: 1.14.16.4) into 5-hydroxytryptophan, which is consequently decarboxylated by L-amino-acid decarboxylase producing 5-HT (Figure 2) [27][28].
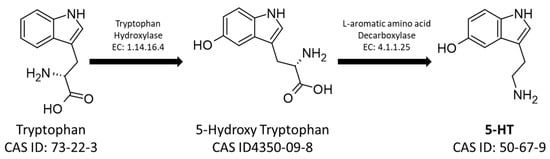
Figure 2. The metabolic pathway for the synthesis of serotonin. The figure shows the CAS-IDs of the molecules along with the EC numbers of the enzymes involved in the biosynthetic pathway.
2. Functional Role of Biogenic Amines in Social Insects
2.1. Reproduction and Castes
An increasing number of works suggest that the switch from the non-reproductive to reproductive phase in social insects is correlated with an upturn titer of dopamine in the individual brain. This variation has been observed in several species, such as honeybees [29], bumblebees [30], and Polistes wasps [31][32]. The potential outcomes of such an increase in dopamine have been delved deeper into honeybees and related to other functions in which this BA is recognized to be involved. For instance, it has been suggested that the higher dopamine content measured in the virgin queens could increase their aggressiveness, thus endowing them with an advantage during fights with the other queens [33][34]. Because dopamine is also known to promote muscle activity and, ultimately, locomotor behavior, high levels of this BA in virgin honeybee queens have also been correlated to the promotion or enhancement of mating flight [35][36].
Peculiar study models are provided by queenless species of ants, in which morphological distinction between the reproductive and the non-reproductive caste is lacking, and a dominance hierarchy maintains the social organization. Through ritualized fights, the dominant females become reproductive while the vast majority of workers remain infertile. Reproductive dominance and hierarchical plasticity proved to be strictly linked to brain neurochemistry, as shown in the ponerine ants Streblognathus peetersi [37]. Unique behavioral patterns allow us to identify three groups of ants in this species: (i) the dominant, called alpha, is the one mating and laying eggs; (ii) the high-rank workers, submitted to the alpha but dominant on (iii) the low-rank workers. The alpha workers were characterized by significantly higher levels of octopamine, while serotonin and dopamine did not consistently vary with the reproductive activity in S. peetersi [37]. As it occurred in honeybees, it is not clear if these BAs variations are strictly linked to the reproductive maturation process or are more devoted to enhancing behavioral changes, such as an increase in aggressiveness, which is needed to foster dominance hierarchies. Both scenarios are likely and not mutually exclusive. Some direct evidence of the role of BAs in ants’ physiological shift to reproduction has been reported by Penick and colleagues [38], that observed an increase in dopamine levels correlated with ovarian activity in Herpegnathos saltator. Indeed, a rise in dopamine content was highlighted when workers with a functional spermatheca, namely gamergates, start reproducing sexually [38][39]. In H. saltator, three types of dopamine receptors were located on the ovary tissues of the gamergates, although the ovarian expression levels of dopaminergic receptor genes were lower than those observed in the brain. Fertility and dominance decreased as the dopamine levels dropped as a consequence of worker policing, a social mechanism used to diminish ovarian activity and to control the number of gamergates. Consequently, a decrease in dopamine may be the first event leading to reduced ovarian activity. Therefore, it is also likely that the queen pheromones used to prevent the development of other queens target similar receptors, eventually decreasing dopamine contents. In Solenopsis invicta, the pheromone released by the queen inhibits the dealation and reproduction of virgin females by acting on dopamine levels. Indeed, the levels of this amine in the brain of virgin females doubled after only 15 days of separation from the queen of the natal colony [40]. In addition to Solenopsis ants [40][41], the honeybee queen pheromone is also known to modulate worker responses by regulating the contents of their BAs, primarily dopamine [42][43].
Unexpectedly, significant differences in the BA levels between queen, male, and worker castes are inconsistent throughout all social insects. For instance, while studying Formica japonica, Aonuma and Watanabe [44] did not find any variation in the average contents of dopamine among castes, while higher titers of this BA were detected in Bombus [45] and honeybee [46] queens than in workers. Interestingly, changes in brain morphology, which can be correlated with variations in BA contents, were shown in males of H. saltator ants [47]. Males possessed a smaller brain than workers, but optic lobes and central complex neuropils were over-developed. In addition, these two regions contained high densities of serotoninergic processes while the rest of the male brain is innervated by fewer aminergic fibers than that of workers. Therefore, in males, serotonin was primarily concentrated in these two brain regions. The peculiar neuroanatomy could provide males with an enhanced ability to perceive visual cues and coordinate movements which are crucial for a successful nuptial flight [47].
Although further corroboration is needed, it is likely a combination of caste interactions, releasing of chemical compounds such as pheromones, but also specific diet (see the royal jelly encompassing precursors of dopamine) [34][48] could act as potential modifiers of BA brain levels (primarily dopamine) which ultimately drive reproductive changes in social insects [41][42][43][44][45][46][49].
2.2. Foraging, Aging, and Labor Division
The hallmark of eusocial species is the strict division of labor among colony members. Temporal polyethism is a common mechanism of functional specialization that consists of splitting tasks based on worker age. In general, the youngest individuals are invested primarily with indoor duties (such as brood care) while, as they get older, they face a gradual transition to extranidial activities. Outdoor tasks are connected with higher risks but also require processing complex or multiple stimuli using more advanced computational, locomotory, and biomechanical capabilities [50][51] and references therein. Variations in BA levels were identified as one of the leading causes of behavioral plasticity and social insect specialization in different tasks. As it occurred in the case of maturation between non-reproductive and reproductive states, most of the studies reported an increase in the content of certain BAs as aging. An upturn in three amines, i.e., dopamine, octopamine, and serotonin, was found in bees [52] and ants [38][39][53] as they grew and developed. However, when Wnuk and collaborators [54] surveyed differences in BAs according to age, task specialization, and behavioral maturation in Formica polyctena, they found that the octopamine levels were significantly higher in nurses, which are younger individuals, than in foragers, being partially in contrast with the aforementioned findings. Overall, F. polyctena proved an interesting model because the division of labor associated with sub-castes in this species can be modified according to the colony requirements. Thus, it is likely to observe a behavioral change in foragers that return to in-nest duties, the so-called “reverted nurses”. Since the amine contents did not differ significantly between “reverted nurses” and foragers, the authors concluded that BAs in this ant species are maturation-related rather than task-related [54]. Nonetheless, a study on Acromyrmex echinatior, a leaf-cutting ant species, established a strong correlation between the division of labor and monoamine brain variations [55]. A. echinatior shows a very complex task specialization with a remarkable morphological distinction between workers. Foragers are bigger ants who leave the nest searching for leaf material to grow their symbiotic fungi, whereas waste-managing workers are smaller individuals that remove leftovers and infectious material from the nest. Foragers showed higher levels of octopamine and dopamine, while serotonin contents were similar to those of waste-managing workers. These results align with studies on other social insects, where octopamine was the main neurotransmitter involved in olfactory memory and outdoor activities [56][57]. High concentrations of octopamine may be related to the foraging activities because they require encoding several olfactory stimuli, like pheromone trails left by nestmates or identifying the food source and quality. Indeed, the involvement of octopamine, and partially of dopamine, in sensing pheromones in the contest of reward learning was also found in Hymenoptera, primary honeybees [58][59][60][61]. Recently, Baracchi and colleagues [61] found that the effect of attractive compounds, such as geraniol, on learning and memory was modulated through octopaminergic and dopaminergic signaling. The most robust evidence of BA involvement in chemical sensing in ants was provided by studying Pheidole dentata. In this species, serotine mediates the forager individual’s response to pheromone trails [62].
Pheromones are not the only signals social insects use while foraging; other cues can be pivotal to finding food resources and returning to the nest. By investigating the foraging behavior of Lasius niger ants, Mannino and co-workers [63] highlighted that the geomagnetic field plays a crucial role in enhancing the workers’ orientation performance. With respect to workers assayed in near-null magnetic field conditions, ants kept in the presence of a normal geomagnetic field showed significantly higher contents of several BAs, which could explain the increase in locomotory (tyramine and dopamine) and chemical perception (octopamine and serotonin) abilities observed during behavioral tests. This work proves that BA modulation could be the molecular mechanism through which the geomagnetic field affects L. niger orientation performance.
2.3. Nestmate Recognition and Aggressive Behavior
In social insects, discriminating between nestmates and non-nestmates is a fundamental process for defending their societies and territories from members of other colonies or species. Within a colony, each individual possesses a layer of several compounds, especially cuticular hydrocarbons, which primarily protect the insect from desiccation, but have become essential communication signals during evolution [6]. Trophallaxis and allogrooming permit the transfer of these compounds among members of the society, allowing to assemble the so-called “colony odor” [64].
Two main hypotheses have been formulated to explain the nestmate discrimination mechanism in ants. The first foresees that each colony member can perceive its own chemical signature, and during the encounter with another individual, it compares with the chemical signature of the encountered subject [65]. The second hypothesis is that ants acquire the odor of their colony and nestmates in a continuous learning process to meet any changes in the chemical profile over time [66]. Regardless of the recognition mechanism, when an individual recognizes another individual as a stranger, they will engage in a series of defensive behaviors [67].
Several studies have highlighted the active involvement of biogenic amines in the intra- and interspecific recognition process and in aggressive reactions. In honeybees, for example, octopamine-treated workers increased aggression toward non-nestmates and reduced aggression toward nestmates, indicating that higher octopamine concentrations improve recognition of nestmates by reducing errors and increasing attention to relevant sensory stimuli [68]. Furthermore, when stimulated with isoamyl acetate, the main component of the alarm pheromone, the levels of two other biogenic amines, serotonin and dopamine, increased, inducing a greater defensive reaction, e.g., stinging behavior [69].
In ants, several biogenic amines appear to be involved in intra- and interspecific recognition processes and aggressive reactions. In Tetramorium caespitum, for instance, changes in octopamine and serotonin in individuals’ brains are sufficient to modify worker ants’ decision to attack non-nestmate individuals, while increases in dopamine levels are associated with physical combat [70]. In workers of Formica aquilonia fed with octopamine, the frequency of attacks toward their natural predators, i.e., ground beetles, increased while the frequency of non-aggressive reactions decreased [71]. In a congeneric species, i.e., F. polyctena, biogenic amine administration was demonstrated to have significant context-dependent behavioral effects, being almost exclusively exhibited in the case of confrontations with allospecific opponents (F. fusca and crickets’ nymphs). Serotonin treatment produced a weak stimulatory effect on aggressive behavior patterns, while dopamine elicited several strong aggressive behaviors toward the opponents. On the contrary, octopamine did not cause any significant effects on the aggressive behaviors of the tested ants, while tyramine administration suppressed threatening behavior directed at F. fusca [72].
The work by Aonuma [73] on Odontomacus kuroiwae shows how levels of biogenic amines can vary at the individual level influencing behavioral patterns related to aggression and defensive responses. Among workers of the trap-jaw ant, less than 10% of individuals tactilely stimulated at the level of the abdomen responded with a rapid turn indicating a higher efficiency in defense behavior than most workers that escape after the stimulus. The levels of octopamine, dopamine, and serotonin in the brain of the workers of the first group were significantly elevated, and the supplementation of serotonin and moderate dopamine contributed to the triggering of defensive responses. A difference in aggressive behavior at the individual level has also been found in Oecophylla smaragdina [74], in which polyphenism exists as in many other ant species; the major workers defend the territory while the minors take care of the offspring. Octopamine modulates aggression in the two sub-castes; levels were significantly higher in major workers, and octopamine was positively correlated with the frequency of aggressive responses to non-nursery mates, indicating that neuromodulators may be associated with size differences and associated specializations.
2.4. Trophallaxis, Feeding, and Interspecific Interactions
Trophallaxis is the exchange of food, nutrients, and other fluids between colony members, i.e., between workers, between workers and queens, and between workers and larvae. It occurs with the exchange of liquids through mouth-to-mouth contact (stomodeal trophallaxis) or anus-mouth (proctodeal trophallaxis). Trophallaxis has an essential role in the establishment of social bonds, in maintaining a balance in the food flow between colony members, and in recruiting new young members of other conspecific colonies [75][76].
In ants, trophallaxis and grooming are typical social behaviors shared among nestmates. In a study by Wada-Katsumata and colleagues [77] on Formica japonica, seven-day food deprivation reduced dopamine levels in workers’ brains, and the starved individuals did not perform trophallaxis toward nestmates. The brain octopamine level tended instead to increase in those individuals who were isolated from nestmates. Normal levels of the two biogenic amines were re-established when the isolated ants were reintroduced into the colony, indicating that social interactions, e.g., trophallaxis and grooming, with nestmates can influence the homeostasis of brain biogenic amines in F. japonica. Similarly, in the ant Camponotus fellah, octopamine reduced trophallaxis in workers, indicating that this molecule could be partly responsible for social cohesion among nestmates in ant colonies [75]. In starved individuals of Formica japonica, Wada-Katsumata and colleagues [77] observed a reduction in the frequency of trophallaxis and dopamine levels. After sucrose feeding, dopamine levels remained low or even decreased. In contrast, hungry subjects restored normal dopamine levels after the trophallaxis occurred, confirming that this particular interaction not only improves social bonding but also helps to modulate stress in individuals, thus maintaining the colony balance.
Another biogenic amine may be involved in the feeding process. Indeed, it seems that serotonin can induce satiety in the Componotus mus ant by lowering the intake of carbohydrates. Individuals given oral serotonin and sucrose showed reduced interest in carbohydrates while preferring to feed on protein solutions [78]. However, it is impossible to say whether this change is caused directly by serotonin itself or by the sucrose solution; thus, further investigations are needed [78].
BAs were shown to play a role also in interspecific interactions. Recently, some associations previously defined as mutualistic have been reviewed as potential cases of host manipulation. An outstanding example is provided by the work of Hojo and colleagues [79], who found that the secretions released by a butterfly caterpillar, Narathura japonica, increased the aggressiveness and reduced the locomotory ability of the host ant Pristomyrmex punctatus by regulating the dopamine levels in the worker’s brain. Therefore, the ants offer protection to the butterfly larvae not because of the nutritional gain provided by their secretions but because through these, they receive manipulative drugs acting on their behavior, eventually enhancing their partner fidelity and guarding.
A similar scenario was described in an aphid-ant system, but here the manipulation occurred in both directions. Lasius japonicus ants received high-quality food rewards by promoting the reproductions of particularly honeydew productive morphs of Macrosiphoniella yomogicola [80]. On the other hand, the aphids controlled host ants by secreting dopamine droplets, increasing their aggressiveness. Therefore, M. yomogicola manipulates ants by means of dopamine to gain better protection [80].
A third example involved the interaction between plants and ants. Studying Myrmica scabrinodis, the researchers found that a blend of Origanum vulgare terpenoids, i.e., carvacrol and thymol, decreased the locomotor activity of ants and increased their aggressiveness by regulating the BA levels in workers’ brains [24]. The release of carvacrol is triggered by the interaction between the plant and Myrmica ants, and this chemical signaling is eavesdropped by females of a myrmecophilous butterfly, Maculinea arion, to locate their optimal egg-laying site [81]. M. arion is an obligate social parasite of Myrmica ants and scent carvacrol to spot Origanum foodplants growing in the foraging range of a Myrmica colony, thus providing the offspring with their necessary host resources. It was unclear why some Myrmica ants keep nesting in the surroundings of Origanum plants, thus increasing their chance of being parasitized by M. arion. The work by Mannino and colleagues [24] suggested that the ant–plant interaction is maintained by the manipulation achieved through the release of Origanum terpenoids which have likely evolved to deter plant enemies directly but could also enhance the plant protection by increasing Myrmica ant fidelity and aggressiveness through brain aminergic regulation.
Interspecific signaling can therefore manipulate the behavior of social insects by influencing their decision-making [82]. However, the diversity of these interactions is so broad that the mechanisms underlying manipulation events are likely to vary significantly within different systems. Still, the aminergic regulation of social insects’ brains remains a promising research scope to fully disentangle the maintenance of these associations.
This entry is adapted from the peer-reviewed paper 10.3390/insects14040386
References
- Wilson, E.O. The Insect Societies; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971; p. 548.
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press: Cambridge, MA, USA, 1990.
- Richards, M.H. Social trait definitions influence evolutionary inferences: A phylogenetic approach to improving social terminology for bees. Curr. Opin. Insect Sci. 2019, 34, 97–104.
- Knapp, R.A.; Norman, V.C.; Rouse, J.L.; Duncan, E.J. Environmentally responsive reproduction: Neuroendocrine signalling and the evolution of eusociality. Curr. Opin. Insect Sci. 2022, 53, 100951.
- Richard, F.J.; Hunt, J.H. Intracolony chemical communication in social insects. Insectes Sociaux 2013, 60, 275–291.
- Barbero, F. Cuticular lipids as a cross-talk among ants, plants and butterflies. Int. J. Mol. Sci. 2016, 17, 1966.
- Hunt, J.H.; Richard, F.J. Intracolony vibroacoustic communication in social insects. Insectes Sociaux 2013, 60, 403–417.
- Casacci, L.P.; Thomas, J.A.; Sala, M.; Treanor, D.; Bonelli, S.; Balletto, E.; Schönrogge, K. Ant pupae employ acoustics to communicate social status in their colony’s hierarchy. Curr. Biol. 2013, 23, 323–327.
- Schönrogge, K.; Barbero, F.; Casacci, L.P.; Settele, J.; Thomas, J.A. Acoustic communication within ant societies and its mimicry by mutualistic and socially parasitic myrmecophiles. Anim. Behav. 2017, 134, 249–256.
- Cervo, R.; Cini, A.; Turillazzi, S. Visual recognition in social wasps. In Social Recognition in Invertebrates: The Knowns and the Unknowns; Aquiloni, L., Tricarico, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–145.
- Sheehan, M.J.; Tibbetts, E.A. Specialized face learning is associated with individual recognition in paper wasps. Science 2012, 334, 1272–1275.
- Leonhardt, S.D.; Menzel, F.; Nehring, V.; Schmitt, T. Ecology and evolution of communication in social insects. Cell 2016, 164, 1277–1287.
- Casacci, L.P.; Bonelli, S.; Balletto, E.; Barbero, F. Multimodal signaling in myrmecophilous butterflies. Front. Ecol. Evol. 2019, 7, 454.
- Casacci, L.P.; Barbero, F.; Ślipiński, P.; Witek, M. The inquiline ant Myrmica karavajevi uses both chemical and vibroacoustic deception mechanisms to integrate into its host colonies. Biology 2021, 10, 654.
- Sasaki, K.; Okada, Y.; Shimoji, H.; Aonuma, H.; Miura, T.; Tsuji, K. Social evolution with decoupling of multiple roles of biogenic amines into different phenotypes in Hymenoptera. Front. Ecol. Evol. 2021, 9, 659160.
- Anton, S.; Rössler, W. Plasticity and modulation of olfactory circuits in insects. Cell Tissue Res. 2021, 383, 149–164.
- Hamilton, A.R.; Shpigler, H.; Bloch, G.; Wheeler, D.E.; Robinson, G.E. Endocrine influences on insect societies. In Hormones, Brain and Behavior; Pfaff, D., Joels, M., Eds.; Academic Press: New York, NY, USA, 2017; pp. 421–451.
- Libersat, F.; Pflueger, H.J. Monoamines and the orchestration of behavior. BioScience 2004, 54, 17–25.
- Roeder, T. Tyramine and octopamine: Ruling behavior and metabolism. Annu. Rev. Entomol. 2005, 50, 447–477.
- Kamhi, J.F.; Traniello, J.F.A. Biogenic amines and collective organization in a superorganism: Neuromodulation of social behavior in ants. Brain. Behav. Evol. 2013, 82, 220–236.
- Kamhi, J.F.; Arganda, S.; Moreau, C.S.; Traniello, J.F.A. Origins of aminergic regulation of behavior in complex insect social systems. Front. Syst. Neurosci. 2017, 11, 74.
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behavior in insects. Annu. Rev. Entomol. 2017, 62, 35–52.
- Downer, R.G.H.; Hiripi, L. Biogenic amines in insects. In Insect Neurochemistry and Neurophysiology 1993; CRC Press: Boca Raton, FL, USA, 2019; pp. 23–38. ISBN 1351073591.
- Mannino, G.; Abdi, G.; Maffei, M.E.; Barbero, F. Origanum vulgare terpenoids modulate Myrmica scabrinodis brain biogenic amines and ant behaviour. PLoS ONE 2018, 13, e0209047.
- Brandau, K.; Axelrod, J. The biosynthesis of octopamine. Naunyn-Schmiedeb. Arch. Pharmacol. 1972, 273, 123–133.
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34.
- Welford, R.W.; Vercauteren, M.; Trébaul, A.; Cattaneo, C.; Eckert, D.; Garzotti, M.; Sieber, P.; Segrestaa, J.; Studer, R.; Groenen, P.M.; et al. Serotonin biosynthesis as a predictive marker of serotonin pharmacodynamics and disease-induced dysregulation. Sci. Rep. 2016, 6, 30059.
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals—An overview. Int. J. Mol. Sci. 2021, 22, 9996.
- Sasaki, K.; Watanabe, T. Sex-specific regulatory systems for dopamine production in the honey bee. Insects 2022, 13, 128.
- Sasaki, K.; Matsuyama, H.; Morita, N.; Ono, M. Caste differences in the association between dopamine and reproduction in the bumble bee Bombus ignitus. J. Insect Physiol. 2017, 103, 107–116.
- Sasaki, K.; Yamasaki, K.; Nagao, T. Neuro-endocrine correlates of ovarian development and egg-laying behaviors in the primitively eusocial wasp (Polistes chinensis). J. Insect Physiol. 2007, 53, 940–949.
- Yoshimura, H.; Yamada, Y.Y.; Sasaki, K. Identification of biogenic amines involved in photoperiod-dependent caste-fate determination during the adult stage in a temperate paper wasp. J. Insect Physiol. 2021, 131, 104223.
- Farkhary, S.I.; Sasaki, K.; Hayashi, S.; Harano, K.; Koyama, S.; Satoh, T. Fighting and stinging responses are affected by a dopamine receptor blocker flupenthixol in honey bee virgin queens. J. Insect Behav. 2017, 30, 717–727.
- Sasaki, K.; Harada, M. Dopamine production in the brain is associated with caste-specific morphology and behavior in an artificial intermediate honey bee caste. PLoS ONE 2020, 15, e0244140.
- Harano, K.; Sasaki, M.; Nagao, T.; Sasaki, K. Dopamine influences locomotor activity in honeybee queens: Implications for a behavioural change after mating. Physiol. Entomol. 2008, 33, 395–399.
- Farkhary, S.I.; Sasaki, K.; Hayashi, S.; Harano, K.; Koyama, S.; Satoh, T. Suppression of flight activity by a dopamine receptor antagonist in honey bee (Apis mellifera) virgin queens and workers. J. Insect Behav. 2019, 32, 218–224.
- Cuvillier-Hot, V.; Lenoir, A. Biogenic amine levels, reproduction and social dominance in the queenless ant Streblognathus peetersi. Sci. Nat. 2006, 93, 149–153.
- Penick, C.A.; Brent, C.S.; Dolezal, K.; Liebig, J. Neurohormonal changes associated with ritualized combat and the formation of a reproductive hierarchy in the ant Harpegnathos saltator. J. Exp. Biol. 2014, 217, 1496–1503.
- Okada, Y.; Sasaki, K.; Miyazaki, S.; Shimoji, H.; Tsuji, K.; Miura, T. Social dominance and reproductive differentiation mediated by dopaminergic signaling in a queenless ant. J. Exp. Biol. 2015, 218, 1091–1098.
- Boulay, R.; Hooper-Bui, L.M.; Woodring, J. Oviposition and oogenesis in virgin fire ant females Solenopsis invicta are associated with a high level of dopamine in the brain. Physiol. Entomol. 2001, 26, 294–299.
- Vander Meer, R.K.; Preston, C.A.; Hefetz, A. Queen regulates biogenic amine level and nestmate recognition in workers of the fire ant, Solenopsis invicta. Sci. Nat. 2008, 95, 1155–1158.
- Beggs, K.T.; Mercer, A.R. Dopamine receptor activation by honey bee queen pheromone. Curr. Biol. 2009, 19, 1206–1209.
- Vergoz, V.; McQuillan, H.J.; Geddes, L.H.; Pullar, K.; Nicholson, B.J.; Paulin, M.G.; Mercer, A.R. Peripheral modulation of worker bee responses to queen mandibular pheromone. Proc. Natl. Acad. Sci. USA 2009, 106, 20930–20935.
- Aonuma, H.; Watanabe, T. Octopaminergic system in the brain controls aggressive motivation in the ant, Formica japonica. Acta Biol. Hung. 2012, 63, 63–68.
- Sasaki, K.; Yokoi, K.; Toga, K. Bumble bee queens activate dopamine production and gene expression in nutritional signaling pathways in the brain. Sci. Rep. 2021, 11, 5526.
- Sasaki, K.; Ugajin, A.; Harano, K. Caste-specific development of the dopaminergic system during metamorphosis in female honey bees. PLoS ONE 2018, 13, e0206624.
- Hoyer, S.C.; Liebig, J.; Rössler, W. Biogenic amines in the ponerine ant Harpegnathos saltator: Serotonin and dopamine immunoreactivity in the brain. Arthropod Struct. Dev. 2005, 34, 429–440.
- Matsuyama, S.; Nagao, T.; Sasaki, K. Consumption of tyrosine in royal jelly increases brain levels of dopamine and tyramine and promotes transition from normal to reproductive workers in queenless honey bee colonies. Gen. Comp. Endocrinol. 2015, 211, 1–8.
- Shimoji, H.; Aonuma, H.; Miura, T.; Tsuji, K.; Sasaki, K.; Okada, Y. Queen contact and among-worker interactions dually suppress worker brain dopamine as a potential regulator of reproduction in an ant. Behav. Ecol. Sociobiol. 2017, 71, 35.
- Maák, I.; Camera, J.; Casacci, L.P.; Barbero, F.; Trigos-Peral, G.; Ślipiński, P.; Bonelli, S.; Zaccagno, M.; Witek, M. The influence of colony traits on the collective behaviour of Myrmica scabrinodis ants. Insect Conserv. Diver. 2019, 12, 481–491.
- Gordon, D.M. The evolution of the algorithms for collective behavior. Cell Syst. 2016, 3, 514–520.
- Schulz, D.J.; Robinson, G.E. Biogenic amines and division of labor in honey bee colonies: Behaviorally related changes in the antennal lobes and age-related changes in the mushroom bodies. J. Comp. Physiol. A 1999, 184, 481–488.
- Seid, M.A.; Traniello, J.F.A. Age-related changes in biogenic amines in individual brains of the ant Pheidole dentata. Sci. Nat. 2005, 92, 198–201.
- Wnuk, A.; Wiater, M.; Godzińska, E.J. Effect of past and present behavioural specialization on brain levels of biogenic amines in workers of the red wood ant Formica polyctena. Physiol. Entomol. 2011, 36, 54–61.
- Smith, A.R.; Muscedere, M.L.; Seid, M.A.; Traniello, J.F.A.; Hughes, W.O.H. BAs are associated with worker task but not patriline in the leaf-cutting ant Acromyrmex echinatior. J. Comp. Physiol. 2013, 199, 1117–1127.
- Schulz, D.J.; Robinson, G.E. Octopamine influences division of labor in honey bee colonies. J. Comp. Physiol. A 2001, 87, 53–61.
- Schulz, D.J.; Sullivan, J.P.; Robinson, G.E. Juvenile hormone and octopamine in the regulation of division of labor in honey bee colonies. Horm. Behav. 2002, 42, 222–231.
- Søvik, E.; Perry, C.J.; Barron, A.B. Insect reward systems: Comparing flies and bees. In Advances in Insect Physiology: Genomics, Physiology and Behaviour of Social Insects; Zayed, A., Kent, C.F., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 189–226.
- Klappenbach, M.; Kaczer, L.; Locatelli, F. Dopamine interferes with appetitive long-term memory formation in honey bees. Neurobiol. Learn. Mem. 2013, 106, 230–237.
- Lenschow, M.; Cordel, M.; Pokorny, T.; Mair, M.M.; Hofferberth, J.; Ruther, J. The post-mating switch in the pheromone response of Nasonia females is mediated by dopamine and can be reversed by appetitive learning. Front. Behav. Neurosci. 2018, 12, 14.
- Baracchi, D.; Cabirol, A.; Devaud, J.M.; Haase, A.; d’Ettorre, P.; Giurfa, M. Pheromone components affect motivation and induce persistent modulation of associative learning and memory in honey bees. Commun. Biol. 2020, 3, 447.
- Muscedere, M.L.; Johnson, N.; Gillis, B.C.; Kamhi, J.F.; Traniello, J.F.A. Serotonin modulates worker responsiveness to trail pheromone in the ant Pheidole dentata. J. Comp. Physiol. A 2012, 198, 219–227.
- Mannino, G.; Casacci, L.P.; Bianco Dolino, G.; Badolato, G.; Maffei, M.E.; Barbero, F. The Geomagnetic Field (GMF) is necessary for black garden ant (Lasius niger L.) foraging and modulates orientation potentially through aminergic regulation and MagR Expression. Int. J. Mol. Sci. 2023, 24, 4387.
- Crozier, R.H.; Dix, M.W. Analysis of two genetic models for the innate components of colony odour in social Hymenoptera. Behav. Ecol. Sociobiol. 1979, 4, 217–224.
- Obin, M.S.; Vander Meer, R.K. Mechanism of template-label matching in fire ant, Solenopsis invicta Buren, nestmate recognition. Anim. Behav. 1989, 38, 430–435.
- Brandstaetter, A.S.; Kleineidam, C.J. Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. J. Neurophysiol. 2011, 106, 2437–2449.
- Menzel, F.; Schmitt, T.; Bluthgen, N. Intraspecific nestmate recognition in two parabiotic ant species: Acquired recognition cues and low inter-colony discrimination. Insectes Sociaux 2009, 56, 251–260.
- Robinson, G.E.; Heuser, L.M.; LeConte, Y.; Lenquette, F.; Hollingworth, R.M. Neurochemicals aid bee nestmate recognition. Nature 1999, 399, 534–535.
- Nouvian, M.; Mandal, S.; Jamme, C.; Claudianos, C.; D’Ettorre, P.; Reinhard, J.; Barron, A.B.; Giurfa, M. Cooperative defence operates by social modulation of biogenic amine levels in the honey bee brain. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172653.
- Bubak, A.N.; Yaeger, J.D.; Renner, K.J.; Swallow, J.G.; Greene, M.J. Neuromodulation of nestmate recognition decisions by pavement ants. PLoS ONE 2016, 11, e0166417.
- Yakovlev, I.K. Effects of octopamine on aggressive behavior in red wood ants. Neurosci. Behav. Physiol. 2018, 48, 279–288.
- Szczuka, A.; Korczyńska, J.; Wnuk, A.; Symonowicz, B.; Szwacka, A.G.; Mazurkiewicz, P.; Kostowski, W.; Godzińska, E.J. The effects of serotonin, dopamine, octopamine and tyramine on behavior of workers of the ant Formica polyctena during dyadic aggression tests. Acta Neurobiol. Exp. 2013, 73, 495–520.
- Aonuma, H. Serotonergic control in initiating defensive responses to unexpected tactile stimuli in the trap-jaw ant Odontomachus kuroiwae. J. Exp. Biol. 2020, 223, jeb228874.
- Kamhi, J.F.; Nunn, K.; Robson, S.K.; Traniello, J.F. Polymorphism and division of labour in a socially complex ant: Neuromodulation of aggression in the Australian weaver ant, Oecophylla smaragdina. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150704.
- Boulay, R.; Soroker, V.; Godziñska, E.J.; Hefetz, A.; Lenoir, A. Octopamine reverses the isolation-induced increase in trophallaxis in the carpenter ant Camponotus fellah. J. Exp. Biol. 2000, 203, 513–520.
- Boulay, R.; Lenoir, A. Social isolation of mature workers affects nestmate recognition in the ant Camponotus fellah. Behav. Process. 2001, 15, 67–73.
- Wada-Katsumata, A.; Yamaoka, R.; Aonuma, H. Social interactions influence dopamine and octopamine homeostasis in the brain of the ant Formica japonica. J. Exp. Biol. 2011, 214, 1707–1713.
- Falibene, A.; Rössler, W.; Josens, R. Serotonin depresses feeding behaviour in ants. J. Insect Physiol. 2012, 58, 7–17.
- Hojo, M.K.; Pierce, N.E.; Tsuji, K. Lycaenid caterpillar secretions manipulate attendant ant behavior. Curr. Biol. 2015, 25, 2260–2264.
- Kudo, T.; Aonuma, H.; Hasegawa, E. A symbiotic aphid selfishly manipulates attending ants via dopamine in honeydew. Sci. Rep. 2021, 11, 18569.
- Patricelli, D.; Barbero, F.; Occhipinti, A.; Bertea, C.M.; Bonelli, S.; Casacci, L.P.; Zebelo, S.A.; Crocoll, C.; Gershenzon, J.; Maffei, M.E.; et al. Plant defences against ants provide a pathway to social parasitism in butterflies. Proc. R. Soc. 2015, 282, 1811.
- Heil, M.; McKey, D. Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 425–453.
This entry is offline, you can click here to edit this entry!
