Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Calendula officinalis Linn. (CO) is a popular medicinal plant from the plant kingdom’s Asteraceae family that has been used for millennia. This plant contains flavonoids, triterpenoids, glycosides, saponins, carotenoids, volatile oil, amino acids, steroids, sterols, and quinines. These chemical constituents confer multifaceted biological effects such as anti-inflammatory, anti-cancer, antihelminthic, antidiabetes, wound healing, hepatoprotective, and antioxidant activities. Additionally, it is employed in cases of certain burns and gastrointestinal, gynecological, ocular, and skin conditions.
- Calendula officinalis
- traditional medicine
- chemical composition
1. Introduction
The use of traditional medicine was found to be first implemented in Ancient Greece. According to Greek traditional knowledge, gods gave the knowledge of healing to man. Theophrastus (372–286 BC), a disciple of Aristotle, an ancient Greek philosopher, and a scientist, authored the first scientific system of plants [1]. Although not wholly aware of their exact physicochemical characteristics at the time, the human population has found additional health benefits from plants throughout history. The same components from plant sources that have a long medicinal history and are proven effective in the welfare of human health are indicated within traditional medicine. This traditional medicinal knowledge and colonial expansion through the progress of communication mediums have been transferred over the generations [1][2]. Currently, traditional medicines are becoming more popular for therapeutic use, specifically for self-treatment practices [3][4][5].
Calendula officinalis Linn. (CO), as an important plant within traditional medicine, has found application in the food industry [6] as well as the pharmaceutical industry [7] owing to the presence of secondary metabolites in the plant. The Calendula genus covers approximately 25 species, among which C. officinalis, C. arvensis, C. tripterocarpa, C. stellata, and C. suffruticose are the most common [8]. CO is the most studied species of Calendula. It has been used medicinally since the 12th century [9][10] and is known as English Marigold, Pot Marigold, Holigold, Mary Bud, Marybud, or Mary Gowles. The name Calendula originates from the Latin term “calends” denoting the first day of each month when the Calendula flower blooms. Along with this, Calendula has also been referred to as the “herb of the sun”, considering the efflorescence of Calendula flowers in the morning and their shriveling in the evening. For a long period, this traditional herb has been used to treat minor burns, wounds, and skin problems. Currently used CO medicines include pot marigold tincture and carophyllenic ointment, which both contain carotenoids derived from the flowers. It is one of the ingredients of the branded homeopathic drug Traumeel®, which is intended to relieve the pain and swelling brought on by sudden musculoskeletal injuries [11]. Moreover, many sources suggest using Calendula petal powder as an economical substitute for saffron because its coloring and flavoring aided in food products in early times [10].
CO is a self-seeding, annual plant species that grows to a height of 12–18 inches and is found near warm and humid atmospheric conditions [12]. A 5 to 7 cm composite flower head rests on the plant’s stem. The flower head consists of an epicalyx of multiple tapered lanceolate sepals, compactly overlayed on each of the two sides by glandular hairs and yellow-orange tubular florets on the interior side [9][13]. CO powder is a yellowish-brown powder with a distinctive aromatic smell and a mildly bitter taste. It contains normocytic stomata in the outer epidermis’ apical region, fragments of the corolla, covering and glandular trichomes, elongated sclerenchymatous cells, fragments of the walls of the ovaries containing brown pigment, pollen grains, fragments of stigma, and fibrous fragments. CO plants are abundantly seen in Central Europe and the Mediterranean regions [14][15]. It is also found in Middle Eastern countries, specifically Cyprus, Turkey, and Iran. In addition, Calendula cultivation has also been observed in India and China on a larger scale [16][17].
It is considered a safe medication when considering its therapeutic potential with a proper dose and other pharmacological indications [9][18]. Some toxicological studies have even proven the safety of acute and subacute administration of Calendula in terms of biochemistry and physical parameters. According to the European Medicines Agency, CO oil is classified as a herbal medical product and has a claimed LD 50 (lethal dose 50) value of 20 mL/kg of body weight [10][19].
2. Therapeutic Applications of Calendula officinalis
Many ailments have been treated with CO; a plant frequently used in homeopathic medicine. Additionally, it can be cytotoxic and inhibit tumor growth [20]. It functions as an antimicrobial [21][22], antioxidant [23], anti-inflammatory [24][25], antiseptic [26], anti-viral [24], hepatoprotective [21], and antidiabetic medicine [27]. It is also applied to the skin to treat various conditions, including inflammation of the skin, open wounds, and laceration wounds that bleed. Additionally, it is used to heal minor ailments such as razor burns and wind burns. The major parts of the CO plant and their therapeutic applications discussed in this research are represented in Figure 1 and Table 1.
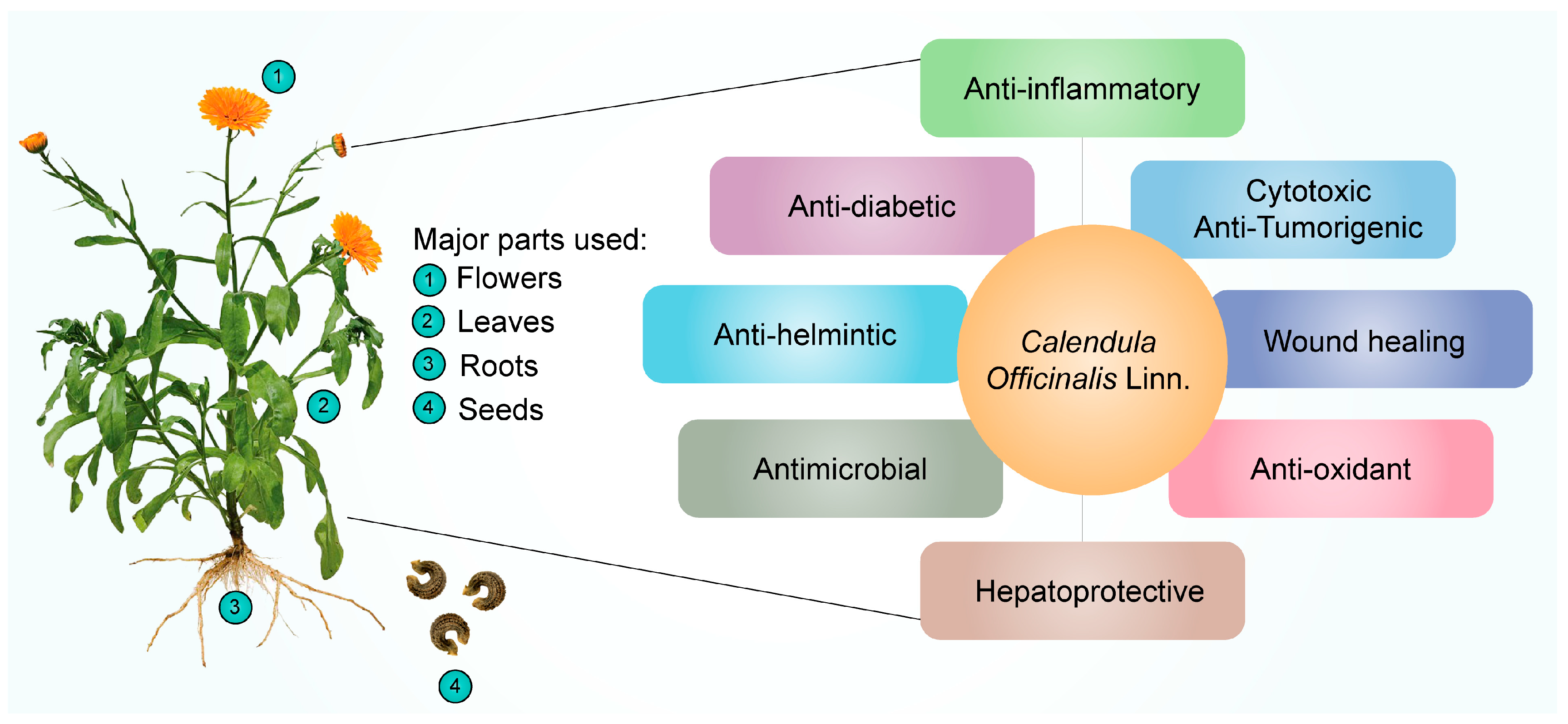
Figure 1. Pharmacological effects of Calendula officinalis Linn.
Table 1. Summary of clinical studies of the use of Calendula officinalis.
| Author and Year | Applicability | Outcomes | Reference |
|---|---|---|---|
| Panahi et al., 2012 | Diaper dermatitis | This study suggests that topical use of CO could be used effectively for the treatment of diaper dermatitis in infants. | [28] |
| Khairnar et al., 2013 | Dental plaque and gingival inflammation | The use of CO mouthwash was able to reduce dental plaque and gingivitis. | [29] |
| Pommier et al., 2004 | Acute dermatitis | Topical use of CO prevented acute dermatitis grade 2 or higher in breast cancer patients given radiation therapy. | [30] |
| Singh and Bagewadi, 2017 | Homogeneous leukoplakia | The use of CO extract gel was effective in reducing the size of the lesion. | [31] |
| Babaee et al., 2013 | Oropharyngeal mucositis | The use of CO extract gel was able to reduce the intensity of oropharyngeal mucositis in patients undergoing radiotherapy during treatment for head and neck cancer. | [32] |
| Giostri et al., 2022 | Acute wounds on hand | CO induced more rapid secondary intention healing in hand and finger wounds | [33] |
| Buzzi et al., 2016 | Venous leg ulcer healing | Patients with ulcers treated with CO extract had a significant 4-fold increase in percentage healing velocity per week, compared with the control group. | [34] |
| De Angelis et al., 2022 | Episiotomy | Women who used CO ointment after episiotomy had significantly lower pain level from the second day and during the entire follow-up. In addition, CO ointment also improves wound healing in terms of redness and edema. | [35] |
| Saffari et al., 2017 | Vaginal candidiasis | Treatment of vaginal candidiasis with CO vaginal lotion seems to be successful. | [36] |
| Pazhohideh et al., 2018 | Bacterial vaginosis | CO was used successfully and without any negative side effects to treat bacterial vaginosis in women of reproductive age. | [37] |
2.1. Anti-Inflammatory
CO is currently being investigated, as it exhibits excellent anti-inflammatory activity. Alkaloids, tannins, flavonoids, essential oils, sterols, saponins, carotenoids, triterpene alcohols, mucilage, polysaccharides, and resin are only a few of the categories of secondary metabolites that the plant has that are correlated with the anti-inflammatory characteristics [38]. Dried flower heads or dried ligulate flowers are plant components that are utilized in medicine and cosmetics. The ligulate flowers are rich in triterpene alcohols, triterpene saponins, fatty acid esters, flavonoids, carotenoids, coumarins, hydrocarbons, essential oils, and fatty acids [39]. Using in vivo pharmacological testing, it has been determined that the triterpenoid fatty acid esters are responsible for the anti-inflammatory effects of Calendula flowers. The lauryl, myristoyl, and palmitoyl esters of faradiol are the most prevalent of these [40], demonstrating that flower extract of CO was much more effective for treating both acute (caused by dextran and carrageenan) and chronic (caused by formalin) swelling in mice. They hypothesized that it may be attributed to the inhibition of the production of proinflammatory cytokines (IL-6, interleukin 6; IL-1β; TNF-α, tumor necrosis factor α; and IFN-γ, interferon γ) and COX-2 (cyclooxygenase 2), and subsequently, Refs. [40][41] demonstrated the anti-inflammatory activity of CO extract and investigated its effects on nitric oxide production. The results revealed that the CO extract inhibited nitric oxide production in a dose-dependent manner, with cytotoxicity only observed at 147 μL/mL concentrations or above.
Garrido-Suárez [25] studied the antinociceptive effects of CO cream on inflammatory hyper-nociception. Rats were subjected to several tests, and it was reported that CO cream (20% or 30% w/w), when applied topically, led to a significant decrease in TNF-α and suppression of COX-2. Pharmaceutical formulations such as nanoemulsion [42] have also been developed to achieve the anti-inflammatory effects of CO. Furthermore, the scientists discovered that all three samples of Calendula extract (3, 5, and 7%) had beneficial effects on healing and soothing wounds when applied to albino rats. The Calendula extract nanoemulsion has an anti-inflammatory impact on skin cells, according to the findings. The schematic representation of the anti-inflammatory effects of CO is shown in Figure 2. The aforementioned information reveals the potential uses of CO as an anti-inflammatory and analgesic agent. Considering this characteristic of CO, it was able to minimize dermatitis in newborns caused by diaper friction when compared to Aloe vera [28]. In the oral cavity, mouth rinsing with CO tincture reduced gingival inflammation [29].
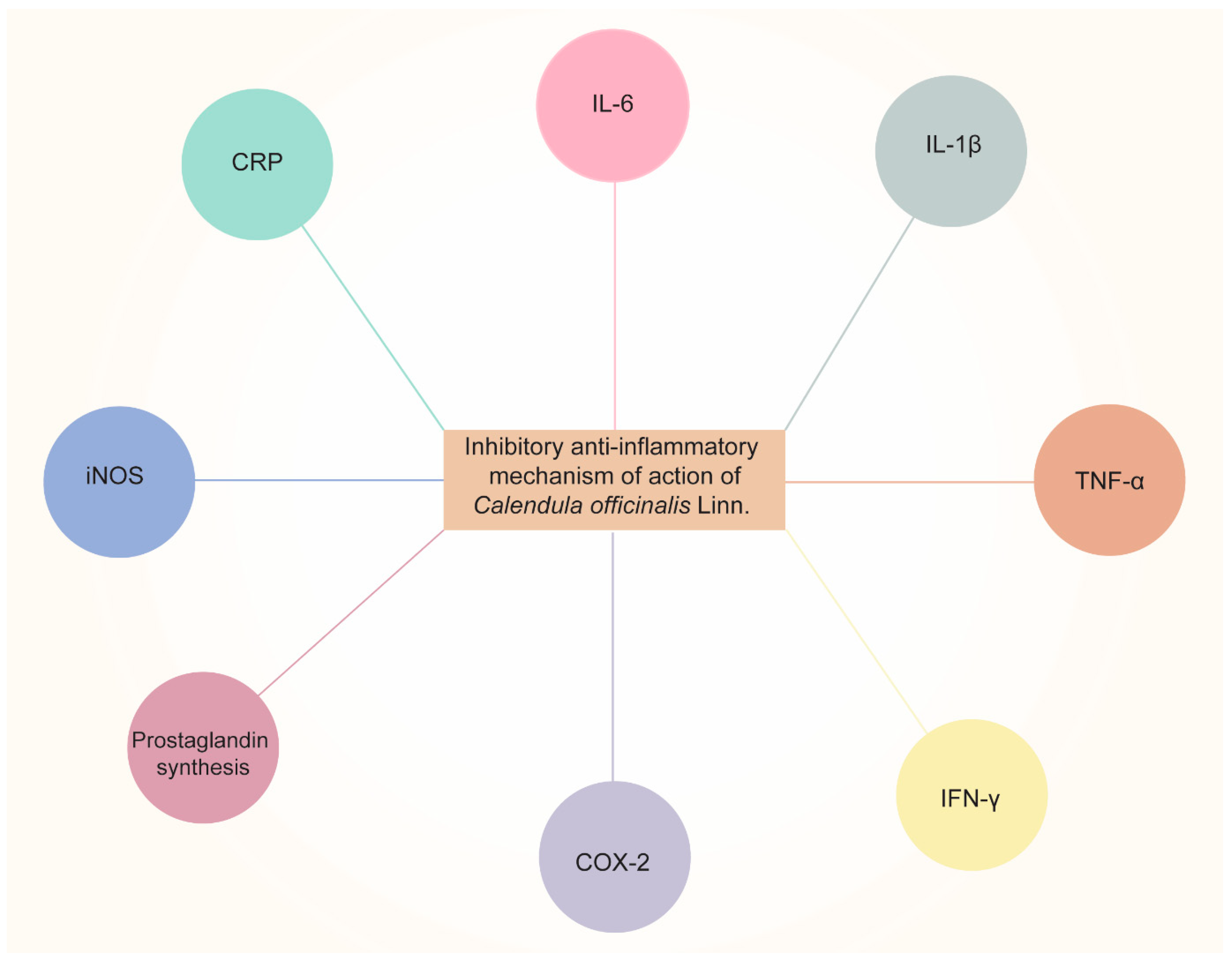
Figure 2. Anti-inflammatory effects of Calendula officinalis Linn by inhibiting pro-inflammatory cytokines (IL-6, IL-1β, TNF-α, and IFN-γ, etc.), COX-2, prostaglandin synthesis, iNOS (inducible nitric oxide synthase), and CRP (C-Reactive Protein).
2.2. Antioxidant Activity
Plant polyphenols such as flavonoids are among the most significant natural compounds with active antioxidant properties. The radical scavenging or chelating flavonoids are caused by their hydroxyl group content [43][44]. The family of antioxidants [43] as phenolic chemicals, on the other hand, operate as free radical terminators [45]. Hence, CO’s high flavonoid and phenolic phytochemical content contribute to its antioxidant activity, which can further promote its strong radical-scavenging capacity and confer protective effects [32]. The leaves and petals of the CO plant contain natural sources of antioxidants [21]. As a result of riboflavin’s photoreduction, it has been claimed that CO extract scavenges hydroxyl and superoxide radicals. Pandey et al. [46] examined the antioxidant properties of the leaves and flowers of CO by using TBA (thiobarbituric acid) and FTC (ferric thiocyanate) techniques. The FTC technique calculated the amount of peroxide produced during the initial stage of linoleic acid peroxidation. The results revealed that the antioxidant concentration decreases with decreasing absorbance value. When compared to regular Vitamins C and E, the aqueous extract of leaves and petals exhibited a high level of antioxidant effect based on absorption rates. The fact that the aqueous extract of the petals displayed lower absorbance with both the FTC and TBA techniques suggests that the petals possessed more antioxidant activity than the leaves.
Based on the evidence, it can be concluded that CO extracts may be extremely beneficial in treating several ailments such as AIDS (acquired immunodeficiency syndrome), heart disease, malaria, diabetes, stroke, cancer, and arteriosclerosis due to their potent antioxidant activity.
2.3. Cytotoxic and Anti-Tumor Activity
Saponin, one of the separated active compounds of CO, has been shown to exhibit antimutagenic action [47]. The interest in the purported anti-tumor activity of CO extracts and components has grown with the rise of complementary and alternative medicine based on herbs as cancer treatment. Cruceriu et al. [48] demonstrated the anti-tumor activity of methanolic extracts of CO using a cell line study. The authors reported that CO extracts could exert anti-cancer activity by inducing apoptosis, activating caspase 3 and caspase 7 at a protein level, and downregulating cyclin D1, D3, A, E, and several cyclin-dependent kinases. Furthermore, BAX (Bcl2 associated X protein) and BBC3 (Bcl2 binding component), two proapoptotic genes, were upregulated and NF-κB (nuclear factor kappa-light-chain enhancer of activated B cells) and STAT3 (signal transducer and activator of transcription factor 3) were downregulated after the treatment with CO extracts. Similarly, Hernández-Rosas et al. [49] demonstrated the in vitro cytotoxic effects of hydro-alcoholic extract of CO on human cancer cell lines. The authors found that the biological activities of high free-radical scavenging capacity (ABTS; 2,2-azino-bis (3-ethylbenzothiazoline-6sulfonic acid, DPPH; 2,2-diphenylpicrylhydrazyl), moderate ability to neutralize hydroxyl radicals, effective metal chelation, and strong reducing capacity are responsible for the anti-cancer effect.
Clinical studies have shown the use of CO in different presentations. At the beginning of the 20th century, the clinical study conducted by Pommier et al. [30] showed the efficacy of Calendula ointment as adjuvant therapy when compared to trolamine for acute dermatitis during irradiation in the treatment of breast cancer. In another study, promising results showed the use of CO gel on oral leukoplakia when compared to lycopene gel [31]. In oral mucositis, the 2% CO mouthwash was able to decrease oral mucositis when compared to the placebo group [32].
In conclusion, there are encouraging findings about CO’s prospective usage in cancer management, particularly in cancer prevention, treatment of cancer, and palliative care for cancer patients. However, progress to pertinent preclinical studies is impeded without understanding the bioactive components responsible for the in vitro and in vivo selective cytotoxicity and for preventing radiotherapy-induced adverse effects. As a result, further study is required to find novel components of CO that have the potential to become useful bioactive components in the treatment of cancer.
2.4. Wound-Healing Activity
Chronic wounds and delayed wound healing are major medical issues that provide difficult clinical challenges for doctors and have profound socioeconomic consequences. Since ancient times, herbs and their preparations have been utilized in addition to traditional medicines to expedite the healing of wounds. In this context, preparations (alcoholic and lipophilic) made from the flowers of CO have received stellar reviews for treating mild skin inflammations and slow-healing wounds. This is accomplished by enhancing the amount of blood and oxygen delivered to the wound site, which encourages the body to produce new tissue. CO plants’ dried petals are used to make tinctures, ointments, and washes to cure mild infections, scrapes, bruises, and burns. CO also contributes to maintaining calmed, hydrated skin by encouraging the development of collagen, a necessary protein for radiant skin.
Deka et al. [26] stated that CO could dramatically increase wound angiogenesis and collagen metabolism, which results in scar softening and emollient characteristics. The floral extract of CO, when applied topically and orally, has therapeutic properties for burns and wounds. An increase in collagen-hydroxyproline and hexosamine shows that the person or animal being treated is mending their wounds. Gunasekaran et al. [50] demonstrated the wound-healing activity of CO in the winter strain of albino rats. The results revealed that a herbal ointment containing CO could inhibit the activation of macrophages and speed up the migration and proliferation of keratinocytes and fibroblasts, which were responsible for wound healing. This was accomplished by preventing the release of proinflammatory cytokines and reducing oxidative stress at the wound site. The mechanism of action of CO for wound healing is shown in Figure 3.
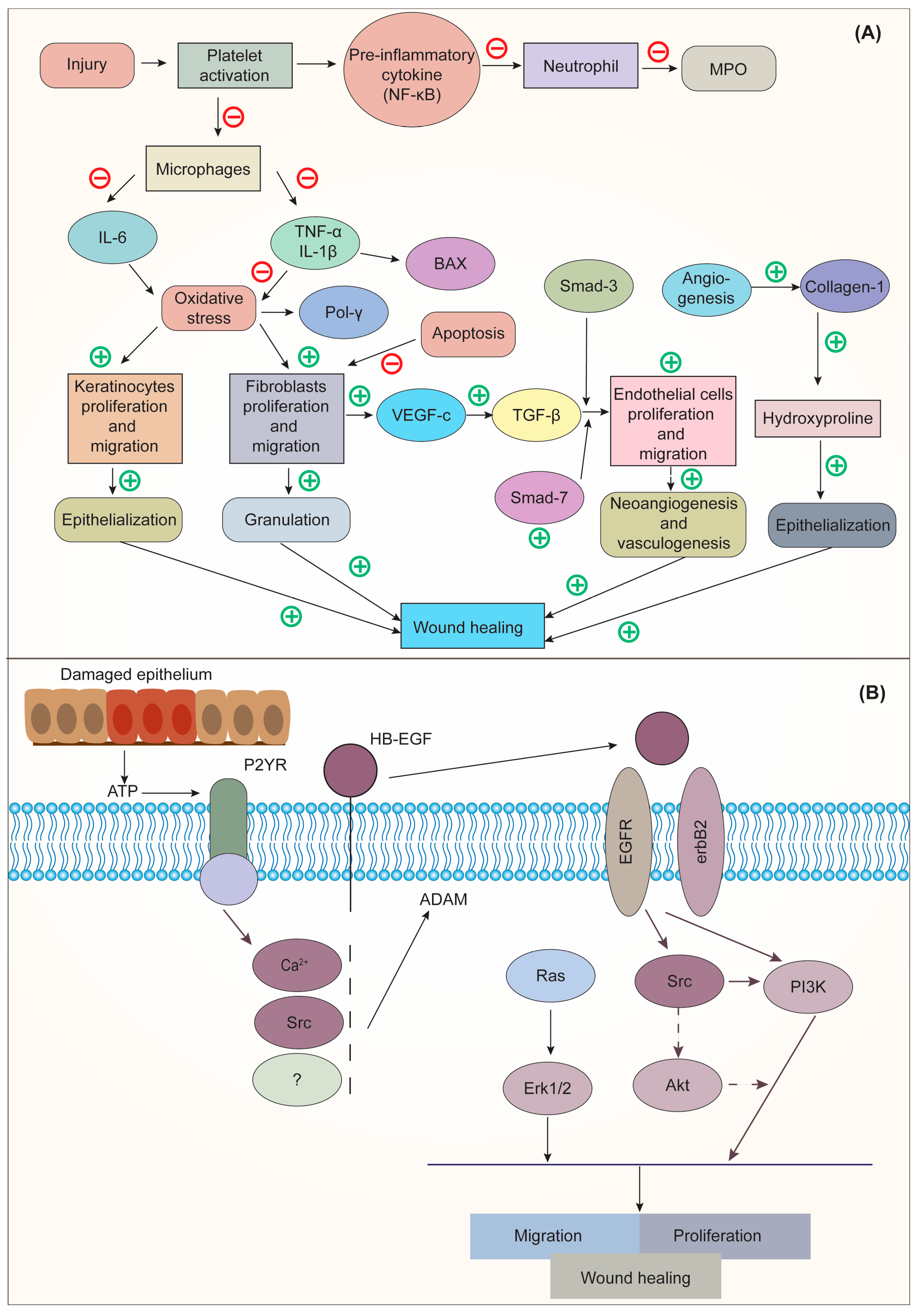
Figure 3. (A). Mechanism of action of CO on Interleukin 6 (IL-6); (B) Mechanism of action of epidermal growth factor (EGF) on wound healing. Adapted from [50] under Creative Commons CC BY license (CC BY 4.0). NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; MPO, myeloperoxidase; IL-6, interleukin-6; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin-1-beta; BAX, BCL-2 associated x protein; Pol γ, DNA polymerase γ; SMAD, suppressor of mothers against decapentaplegic; VEGF-c, vascular endothelial growth factor C; TGF-β, transforming growth factor-beta; ATP, adenosine triphosphate; P2YR, purinergic G protein-coupled receptors; HB-EGF, heparin-binding EGF-like growth factor; EGFR, epidermal growth factor receptor; RAS, rat sarcoma; ERK1/2, extracellular signal-regulated kinase; Src, steroid receptor coactivator; Akt, protein kinase B; PI3K, phosphoinositide 3-kinase.
Similarly, Rathod and co-workers [51] investigated the wound-healing efficacy of CO-loaded collagen films on wounds induced in Wistar rats. On day 21, the rate of wound contraction in the developed CO film was considerably higher than in the control group, the placebo-treated group, and the marketed-product-treated group. In a randomized controlled trial, the CO-containing ointment was studied on 72 qualified primiparous females for cesarean wound healing. According to the findings, applying CO ointment to the wound after a cesarean significantly boosted the rate of wound healing. It can be successfully employed to speed up the cesarean healing process [52].
It is important to note that clinical studies have already been conducted in order to evaluate the efficacy of the use of CO in the healing of hand and finger wounds by secondary intention. In this perspective, there is evidence showing that CO extract is favorable for the treatment of these wounds by reducing the epithelialization time and increasing the healing speed [33]. In chronic wounds, such as venous ulcers, the use of CO also obtained positive results, showing that the treatment with topical CO reduces the surface area of the lesion, achieves greater epithelialization in less time, and accelerates healing time [34]. In addition, this type of healing is advantageous because it reduces medical interventions and treatment costs [53]. Another important finding is that the use of CO ointment after episiotomy reduces pain, redness, and swelling and helps healing [35].
2.5. Hepatoprotective Activity
Most substances that enter the body are processed by the liver, which is also in charge of detoxification. Up to 83% of all pathological cases worldwide are hepatotoxic, making it the most prevalent disease. The main causes of liver toxicity include hepatitis, viral infections, dietary additives, alcohol, toxic industrial chemicals, air pollution, and water pollution. Researchers have shown that CO extracts can protect the liver from the cytotoxicity and oxidative stress caused by carbon tetrachloride. This results in a rise in the amount of total hemoglobin. Similarly, in vitro and in vivo models of the flowers’ hydro-alcoholic extract show decreased hepato-cytolysis and liver biomarkers. The treatment with ethanolic extract brought back normal levels of hepatic blood markers, increased the level of total thiols, decreased levels of total antioxidant status, decreased levels of antioxidant enzymes (CAT, catalase; SOD, superoxide dismutase; GPx, glutathione peroxidase; and GST, glutathione s-transferases) and decreased the levels of malondialdehyde and total oxidant status in both the blood and the hepatocytes. Furthermore, restoration of cellular antioxidant levels, specifically enhanced levels of reduced glutathione enzymatic components and total thiols of the antioxidant system, was also observed, which may be due to the polyphenolic chemicals in CO that protect the cells from chemically induced cellular damage. Moreover, in a dose-dependent manner, CO extract improved the histological picture of the liver, as well as the biochemical parameters and inflammatory cytokines [54].
2.6. Anthelmintic Activity
In addition to being a major cause of illness in humans and animals, parasitic infections also negatively impact the economy. Due to increased resistance to conventional antihelminthic treatments, there has been a quantum leap toward investigating herbal medicines. Herbs such as CO have been used for centuries to combat parasitic illnesses, and they are still utilized for that purpose in many countries. In a study, Khursheed et al. [24] investigated the anthelmintic activity in adult Indian earthworms (Pheretima posthuma). It was observed that the ethanolic extracts of CO exhibited anthelmintic activity (paralysis of the worms followed by death) at 10 mg/mL concentration compared with the standard drug, albendazole. CO was also proven to show anthelminthic activity against Ascaris suum [55] and 50% efficacy on L1-2 larvae of Strongiloides papillosus [56].
2.7. Antimicrobial Activity
Although antibiotics have played a significant part in the treatment of infectious diseases caused by bacteria and fungi for the past 60 years, it has been observed that the occurrence of dangerous bacteria that are resistant to antibiotics has increased in frequency over the course of the past several decades [57]. Because there are a number of different mechanisms by which drug resistance can be manifested, finding a solution to this issue is not likely to be an easy challenge. Because of the growing prevalence of drug-resistant pathogens, there is an immediate and pressing requirement to discover and isolate new bioactive compounds derived from medicinal plants using standardized and contemporary analytical methods. Compounds obtained from medicinal plants might provide unique and relatively simple techniques to treat pathogenic microbes. CO extracts have also proven to be effective as antimicrobial agents [58].
This entry is adapted from the peer-reviewed paper 10.3390/ph16040611
References
- Knoess, W.; Wiesner, J. The Globalization of Traditional Medicines: Perspectives Related to the European Union Regulatory Environment. Engineering 2019, 5, 22–31.
- Galucio, N.C.d.R.; Moysés, D.d.A.; Pina, J.R.S.; Marinho, P.S.B.; Gomes Júnior, P.C.; Cruz, J.N.; Vale, V.V.; Khayat, A.S.; Marinho, A.M.d.R. Antiproliferative, Genotoxic Activities and Quantification of Extracts and Cucurbitacin B Obtained from Luffa operculata (L.) Cogn. Arab. J. Chem. 2022, 15, 103589.
- Chandorkar, N.; Tambe, S.; Amin, P.; Madankar, C. A Systematic and Comprehensive Review on Current Understanding of the Pharmacological Actions, Molecular Mechanisms, and Clinical Implications of the Genus Eucalyptus. Phytomed. Plus 2021, 1, 100089.
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.D.A.; de Oliveira, M.S. Recent Trends in the Application of Essential Oils: The next Generation of Food Preservation and Food Packaging. Trends Food Sci. Technol. 2022, 129, 421–439.
- Muzammil, S.; Neves Cruz, J.; Mumtaz, R.; Rasul, I.; Hayat, S.; Khan, M.A.; Khan, A.M.; Ijaz, M.U.; Lima, R.R.; Zubair, M. Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa Oleifera Leaf Extracts. Molecules 2023, 28, 710.
- Savic Gajic, I.M.; Savic, I.M.; Skrba, M.; Dosić, A.; Vujadinovic, D. Food Additive Based on the Encapsulated Pot Marigold (Calendula officinalis L.) Flowers Extract in Calcium Alginate Microparticles. J. Food Process. Preserv. 2022, 46, e15792.
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and Characterization of Calendula officinalis-Loaded PCL/Gum Arabic Nanocomposite Scaffolds for Wound Healing Applications. Iran. Polym. J. 2019, 28, 51–63.
- Fallahi, M.; Mohammadi, A.; Miri, S.M. The Natural Variation in Six Populations of Calendula officinalis L.: A Karyotype Study. J. Genet. 2020, 6, 34–40.
- Basch, E.; Bent, S.; Foppa, I.; Haskmi, S.; Kroll, D.; Mele, M.; Szapary, P.; Ulbricht, C.; Vora, M.; Yong, S. Marigold (Calendula officinalis L.): An Evidence-Based Systematic Review by the Natural Standard Research Collaboration. J. Herb. Pharm. 2006, 6, 135–159.
- Gu, J.; Aidy, A.; Goorani, S. Anti-Human Lung Adenocarcinoma, Cytotoxicity, and Antioxidant Potentials of Copper Nanoparticles Green-Synthesized by Calendula officinalis. J. Exp. Nanosci. 2022, 17, 285–296.
- Schneider, C. Traumeel – an Emerging Option to Nonsteroidal Anti-Inflammatory Drugs in the Management of Acute Musculoskeletal Injuries. Int. J. Gen. Med. 2011, 4, 225–234.
- Ashwlayan, V.D.; Kumar, A.; Verma, M.; Garg, V.K.; Gupta, S. Therapeutic Potential of Calendula officinalis. Pharm. Pharmacol. Int. J. 2018, 6, 1.
- Almeida, V.M.; Dias, Ê.R.; Souza, B.C.; Cruz, J.N.; Santos, C.B.R.; Leite, F.H.A.; Queiroz, R.F.; Branco, A. Methoxylated Flavonols from Vellozia Dasypus Seub Ethyl Acetate Active Myeloperoxidase Extract: In Vitro and in Silico Assays. J. Biomol. Struct. Dyn 2022, 40, 7574–7583.
- Arora, D.; Rani, A.; Sharma, A. A Review on Phytochemistry and Ethnopharmacological Aspects of Genus Calendula. Pharmacogn. Rev. 2013, 7, 179–187.
- Rego, C.M.A.; Francisco, A.F.; Boeno, C.N.; Paloschi, M.V.; Lopes, J.A.; Silva, M.D.S.; Santana, H.M.; Serrath, S.N.; Rodrigues, J.E.; Lemos, C.T.L.; et al. Inflammasome NLRP3 Activation Induced by Convulxin, a C-Type Lectin-like Isolated from Crotalus Durissus Terrificus Snake Venom. Sci. Rep. 2022, 12, 1428.
- Ercetin, T.; Senol, F.S.; Erdogan Orhan, I.; Toker, G. Comparative Assessment of Antioxidant and Cholinesterase Inhibitory Properties of the Marigold Extracts from Calendula arvensis L. and Calendula officinalis L. Ind. Crop. Prod. 2012, 36, 203–208.
- Gonçalves, A.C.; Castro, S.; Paiva, J.; Santos, C.; Silveira, P. Taxonomic Revision of the Genus Calendula (Asteraceae) in the Iberian Peninsula and the Balearic Islands. Phytotaxa 2018, 352, 1–91.
- Medical Economics Company. PDR for Herbal Medicines; Medical Economics Co.: Montvale, NJ, USA, 2000; ISBN 1563633612.
- R Silva, E.J.; Gonçalves, E.S.; Aguiar, F.; Evêncio, L.B.; A Lyra, M.M.; Cristina C Coelho, M.O.; do Carmo A Fraga, M.C.; Wanderley, A.G.; Gonçalves Wanderley, A.; Rego, M. Toxicological Studies on Hydroalcohol Extract of Calendula officinalis L. Phytother. Res. 2007, 21, 332–336.
- Jiménez-Medina, E.; Garcia-Lora, A.; Paco, L.; Algarra, I.; Collado, A.; Garrido, F. A New Extract of the Plant Calendula officinalis Produces a Dual in Vitro Effect: Cytotoxic Anti-Tumor Activity and Lymphocyte Activation. BMC Cancer 2006, 6, 119.
- Patil, K.; Sanjay, C.; Doggalli, N.; Devi, K.R.; Harshitha, N. A Review of Calendula officinalis Magic in Science. J. Clin. Diagn. Res. 2022, 16, ZE23–ZE27.
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainabl–Green Synthesis of Silver Nanoparticles Using Aqueous Hyssopus Officinalis and Calendula officinalis Extracts and Their Antioxidant and Antibacterial Activities. Molecules 2022, 27, 7700.
- Vinola, S.M.J.; Sekar, M.; Renganathan, S.K.; Dhiraviam, S. Comparative Evaluation of Calendula officinalis and 2% Chlorhexidine against Enterococcus Faecalis and Candida Albicans. J. Interdiscip. Dent. 2022, 11, 119.
- Khursheed, A.; Devender, P.; Shahid, A.; Vandana Arora, S.; Alok, B.; Alam Professor, K. Evaluation of Anthelmintic Activity of Calendula officinalis Flowers Extract. J. Drug Deliv. Ther. 2021, 11, 48–50.
- Garrido-Suárez, B.B.; Garrido, G.; Menéndez, A.B.; Merino, N.; Valdés, O.; de la Paz, N.; Romero, A.; Delgado, L.; Fernández, M.D.; Piñeros, O.; et al. Topical Calendula officinalis L. Inhibits Inflammatory Pain through Antioxidant, Anti-Inflammatory and Peripheral Opioid Mechanisms. J. Integr. Med. 2022, 21, 34–46.
- Deka, B.; Bhattacharjee, B.; Shakya, A.; Ikbal, A.M.A.; Goswami, C.; Sarma, S. Mechanism of Action of Wound Healing Activity of Calendula officinalis: A Comprehensive Review. Pharm. Biosci. J. 2021, 9, 28–44.
- Hao, W.; Jia, Y.; Wang, C.; Wang, X. Preparation, Chemical Characterization and Determination of the Antioxidant, Cytotoxicity and Therapeutic Effects of Gold Nanoparticles Green-Synthesized by Calendula officinalis Flower Extract in Diabetes-Induced Cardiac Dysfunction in Rat. Inorg. Chem. Commun. 2022, 144, 109931.
- Panahi, Y.; Sharif, M.R.; Sharif, A.; Beiraghdar, F.; Zahiri, Z.; Amirchoopani, G.; Marzony, E.T.; Sahebkar, A. A Randomized Comparative Trial on the Therapeutic Efficacy of Topical Aloe Vera and Calendula officinalis on Diaper Dermatitis in Children. Sci. World J. 2012, 2012, 810234.
- Khairnar, M.S.; Pawar, B.; Marawar, P.P.; Mani, A. Evaluation of Calendula officinalis as an Anti-Plaque and Anti-Gingivitis Agent. J. Indian Soc. Periodontol. 2013, 17, 741.
- Pommier, P.; Gomez, F.; Sunyach, M.P.; D’Hombres, A.; Carrie, C.; Montbarbon, X. Phase III Randomized Trial of Calendula officinalis Compared with Trolamine for the Prevention of Acute Dermatitis during Irradiation for Breast Cancer. J. Clin. Oncol. 2004, 22, 1447–1453.
- Singh, M.; Bagewadi, A. Comparison of Effectiveness of Calendula officinalis Extract Gel with Lycopene Gel for Treatment of Tobacco-Induced Homogeneous Leukoplakia: A Randomized Clinical Trial. Int. J. Pharm. Investig. 2017, 7, 88.
- Babaee, N.; Moslemi, D.; Khalilpour, M.; Vejdani, F.; Moghadamnia, Y.; Bijani, A.; Baradaran, M.; Kazemi, M.T.; Khalilpour, A.; Pouramir, M.; et al. Antioxidant Capacity of Calendula officinalis Flowers Extract and Prevention of Radiation Induced Oropharyngeal Mucositis in Patients with Head and Neck Cancers: A Randomized Controlled Clinical Study. DARU J. Pharm. Sci. 2013, 21, 18.
- Giostri, G.S.; Novak, E.M.; Buzzi, M.; Guarita-Souza, L.C. Treatment of Acute Wounds in Hand with Calendula officinalis L.: A Randomized Trial. Tissue Barriers 2021, 10, 1994822.
- Buzzi, M.; De Freitas, F.; De Barros Winter, M. Therapeutic Effectiveness of a Calendula officinalis Extract in Venous Leg Ulcer Healing. J. Wound Care 2016, 25, 732–739.
- De Angelis, C.; Di Stadio, A.; Vitale, S.; Saccone, G.; De Angelis, M.C.; Zizolfi, B.; Di Spiezio Sardo, A. Use of Calendula Ointment after Episiotomy: A Randomized Clinical Trial. J. Matern. Fetal Neonatal Med. 2020, 35, 1860–1864.
- Saffari, E.; Mohammad-Alizadeh-Charandabi, S.; Adibpour, M.; Mirghafourvand, M.; Javadzadeh, Y. Comparing the Effects of Calendula officinalis and Clotrimazole on Vaginal Candidiasis: A Randomized Controlled Trial. Women Health 2016, 57, 1145–1160.
- Pazhohideh, Z.; Mohammadi, S.; Bahrami, N.; Mojab, F.; Abedi, P.; Maraghi, E. The Effect of Calendula officinalis versus Metronidazole on Bacterial Vaginosis in Women: A Double-Blind Randomized Controlled Trial. J. Adv. Pharm. Technol. Res. 2018, 9, 19.
- Fonseca, Y.M.; Catini, C.D.; Vicentini, F.T.M.C.; Nomizo, A.; Gerlach, R.F.; Fonseca, M.J.V. Protective Effect of Calendula officinalis Extract against UVB-Induced Oxidative Stress in Skin: Evaluation of Reduced Glutathione Levels and Matrix Metalloproteinase Secretion. J. Ethnopharmacol. 2010, 127, 596–601.
- Hamburger, M.; Adler, S.; Baumann, D.; Förg, A.; Weinreich, B. Preparative Purification of the Major Anti-Inflammatory Triterpenoid Esters from Marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338.
- Silva, D.; Ferreira, M.S.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Anti-Inflammatory Activity of Calendula officinalis L. Flower Extract. Cosmetics 2021, 8, 31.
- Dennis Bilavendran, J.; Manikandan, A.; Thangarasu, P.; Sivakumar, K. Synthesis and Discovery of Pyrazolo-Pyridine Analogs as Inflammation Medications through pro- and Anti-Inflammatory Cytokine and COX-2 Inhibition Assessments. Bioorg. Chem. 2020, 94, 103484.
- Kiaei, N.; Hajimohammadi, R.; Hosseini, M. Investigation of the Anti-Inflammatory Properties of Calendula Nanoemulsion on Skin Cells. Bioinspired Biomim. Nanobiomater. 2018, 7, 228–237.
- Cook, N.C.; Samman, S. Flavonoids--Chemistry, Metabolism, Cardioprotective Effects, and Dietary Sources. J. Nutr. Biochem. 1996, 7, 66–76.
- Younes, M.; Siegers, C.P. Inhibitory Action of Some Flavonoids on Enhanced Spontaneous Lipid Peroxidation Following Glutathione Depletion. Planta Med. 1981, 43, 240–244.
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Correlations of Antioxidant Activity against Phenolic Content Revisited: A New Approach in Data Analysis for Food and Medicinal Plants. J. Food Sci. 2009, 74, R107–R113.
- Pandey, P.; Despande, B. Antioxidant Activity in the Leaves and Petals of Calendula officinalis Linn. Asian Pac. J. Health Sci. 2022, 9, 130–132.
- Elias, R.; De Méo, M.; Vidal-Ollivier, E.; Laget, M.; Balansard, G.; Dumenil, G. Antimutagenic Activity of Some Saponins Isolated from Calendula officinalis L., C. arvensis L. and Hedera helix L. Mutagenesis 1990, 5, 327–332.
- Cruceriu, D.; Balacescu, O.; Rakosy, E. Calendula officinalis: Potential Roles in Cancer Treatment and Palliative Care. Integr. Cancer Ther. 2018, 17, 1068–1078.
- Hernández-Rosas, N.A.; García-Zebadúa, J.C.; Hernández-Delgado, N.; Torres-Castillo, S.; Figueroa-Arredondo, P.; Mora-Escobedo, R.; Hernández-Rosas, N.A.; García-Zebadúa, J.C.; Hernández-Delgado, N.; Torres-Castillo, S.; et al. Polyphenols Profile, Antioxidant Capacity, and in Vitro Cytotoxic Effect on Human Cancer Cell Lines of a Hydro-Alcoholic Extract from Calendula officinalis L. Petals. TIP Rev. Espec. Cienc. Quím.-Biol. 2018, 21, 54–64.
- Gunasekaran, S.; Arul, A.; Nayagam, J.; Natarajan, R. Wound Healing Potentials of Herbal Ointment Containing Calendula officinalis Linn. on the Alteration of Immunological Markers and Biochemical Parameters in Excision Wounded Animals. Clin. Phytosci. 2020, 6, 77.
- Rathod, L.; Bhowmick, S.; Patel, P.; Sawant, K. Calendula Flower Extract Loaded Collagen Film Exhibits Superior Wound Healing Potential: Preparation, Evaluation, in-Vitro & in-Vivo Wound Healing Study. J. Drug Deliv. Sci. Technol. 2022, 72, 103363.
- Jahdi, F.; Khabbaz, A.H.; Kashian, M.; Taghizadeh, M.; Haghani, H. The Impact of Calendula Ointment on Cesarean Wound Healing: A Randomized Controlled Clinical Trial. J. Fam. Med. Prim. Care 2018, 7, 893.
- Bosley, R.; Leithauser, L.; Turner, M.; Gloster, H.M. The Efficacy of Second-Intention Healing in the Management of Defects on the Dorsal Surface of the Hands and Fingers after Mohs Micrographic Surgery. Dermatol. Surg. 2012, 38, 647–653.
- Pawan, V.; Rajinder, R.; Maninder, S.; Wazir, V.S. Kumar Pawan Attenuating Potential of Calendula officinalis on Biochemical and Antioxidant Parameters in Hepatotoxic Rats. Indian J Physiol. Pharmacol. 2017, 61, 398–410.
- Băieş, M.H.; Gherman, C.; Boros, Z.; Olah, D.; Vlase, A.M.; Cozma-Petruț, A.; Györke, A.; Miere, D.; Vlase, L.; Crișan, G.; et al. The Effects of Allium sativum L., Artemisia absinthium L., Cucurbita pepo L., Coriandrum sativum L., Satureja hortensis L. and Calendula officinalis L. on the Embryogenesis of Ascaris Suum Eggs during an In Vitro Experimental Study. Pathogens 2022, 11, 1065.
- Boyko, O.; Brygadyrenko, V. Nematicidal Activity of Essential Oils of Medicinal Plants. Folia Oecologica 2021, 48, 42–48.
- Rafał, I.G.; Króliczewski, B.J.; Górniak, I.; Bartoszewski, R.; Króliczewski, Á.J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2018, 18, 241–272.
- Khalid, K.A.; EL-Ghorab, A.H. The Effect of Presowing Low Temperature on Essential Oil Content and Chemical Composition of Calendula officinalis. J. Essent. Oil Bear. Plants 2013, 9, 32–41.
This entry is offline, you can click here to edit this entry!
