Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Neonatal sepsis is a major cause of morbidity and mortality in preterm infants.
- preterm
- microbiome
- oxidative stress
1. Introduction
The incidence of early-onset sepsis (EOS) increases as gestational age decreases, with up to 6 cases per 1000 infants born at <34 weeks’ gestation or 20 cases per 1000 infants born at <29 weeks’ gestation. Moreover, preterm infants suffer high rates of EOS-attributable mortality (as high as 40% among extremely low gestation infants). If researchers take birth weight into account, the incidence of EOS in VLBW varies from 9 to 11 cases per 1000 infants [1][2].
These rates increase in case of late-onset sepsis (LOS), where are estimated to be around 12–28% among 22 to 26 weeks’ preterm infants, with a mortality close to 35% in the most vulnerable, lowest-gestation infants [3][4].
Remarkably, signs and symptoms of neonatal sepsis are subtle and nonspecific and frequently undistinguishable from clinical characteristics of preterm infants during postnatal adaptation, rendering clinical diagnosis extremely difficult. Moreover, consensus definition is still lacking [5][6].
The gold standard for the diagnosis of sepsis is a positive blood culture with an identified microorganism. However, despite the use of innovative laboratory methods, results are often delayed more than 24 h or do not yield conclusive results for the clinician. Various circumstances such as low bacteriemia, small blood volume inoculation or antibiotics administered to the mother often hamper achieving prompt and reliable results [7].
Recently, the use of multiplex polymerase chain reaction and molecular assays based on microbial genome hybridization or amplification have been tested in an attempt to detect the presence of bacteria early in the course of disease [8].
However, these techniques need a period of incubation that ranges from 4 to 6 h and a high blood volume to improve sensitivity. Moreover, bacterial PCR assays do not discriminate between viable and nonviable organisms or free and cell-associated DNA, and they do not provide enough information about antibiotic resistance [9]. For these reasons, some authors prefer to use the traditional blood culture better than these alternative methods [10].
The availability of a rapid and reliable diagnostic tool for neonatal sepsis still represents a major challenge for clinicians. It would allow not only early identification of infected infants and improve outcomes but also reduced exposure to unnecessary antimicrobials, subsequently avoiding the risk of inducing antibiotic resistance [11]. Furthermore, antibiotics indiscriminately affect all the commensal gut microbial ecosystem and can, therefore, alter its composition [12]. Finally, the use of antibiotics in the absence of sepsis has been related to an increased risk of mortality or major morbidity such as persistent periventricular echogenicity or echolucency on neuroimaging, chronic lung disease, and stage 3 or higher retinopathy of prematurity [13].
Since sepsis is a widespread process that is not confined to any specific organ, most studies have investigated the role of several mediators of the inflammatory cascade in the early diagnosis of sepsis. Some diagnostic biomarkers based on the immune response such as acute phase reactant proteins, procalcitonin, cytokines or cell surface antigens have been proposed to early detect bacterial sepsis [14][15] and/or to monitor response to therapy [16]. However, no single biomarker so far has shown enough diagnostic power to rule out sepsis at the time of clinical suspicion so far and, therefore, the concurrent use of several biomarkers in a sequential manner and based on immune response to achieve higher sensitivity and specificity has been recommended [17]. These immune and inflammatory responses triggered by the sepsis release proteases, cytotoxic enzymes, reactive oxygen and nitrogen species that could lead to oxidative stress and tissular damage. The pro-oxidant environment linked to the mediators of inflammation is capable of producing dysbiosis and changes in the gut microbiome (Figure 1). A global approach based on the knowledge of immune response and secondary induced changes in metabolomic and microbiome profiles should go in depth for a better management of this issue.
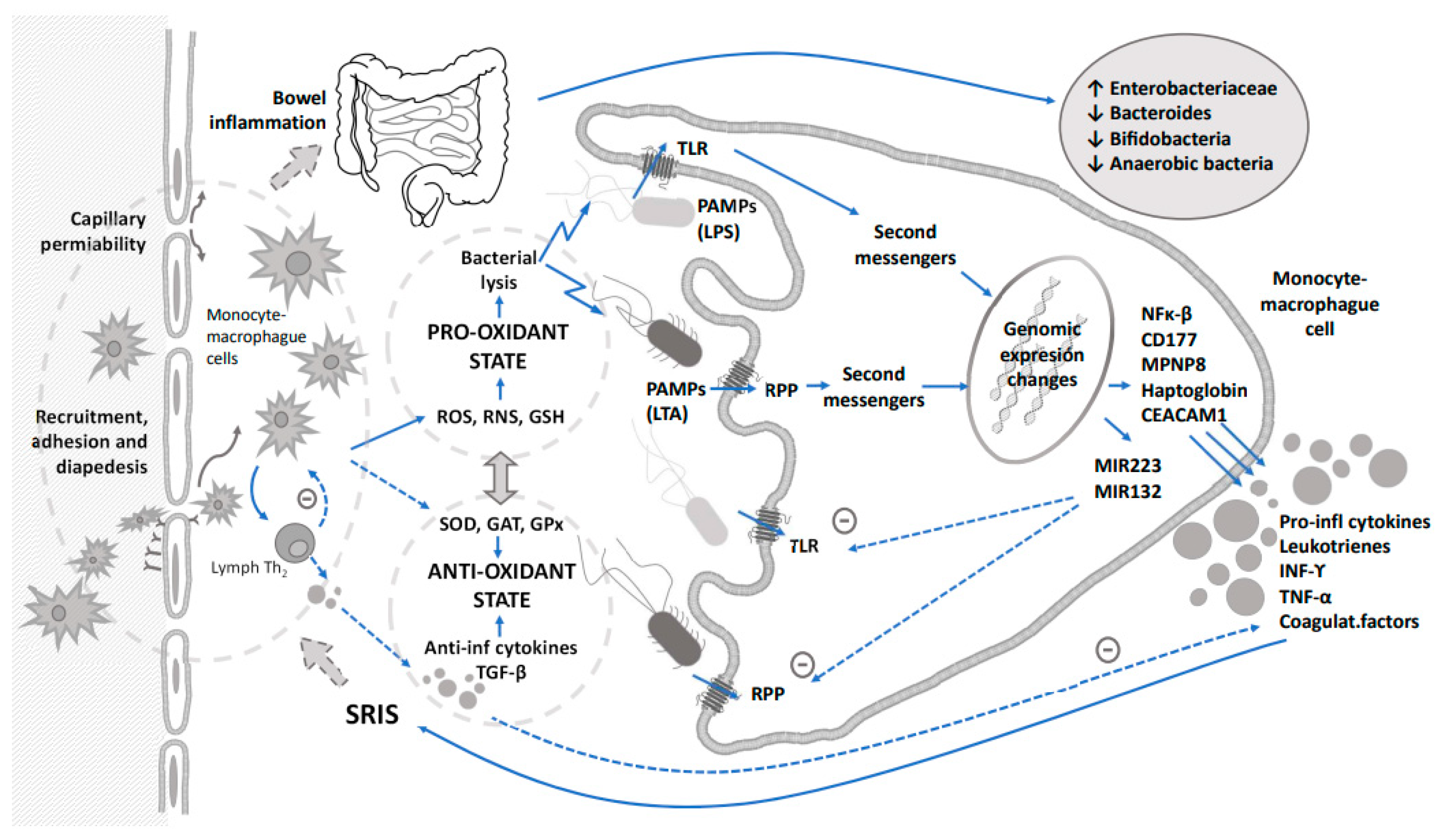
Figure 1. Integrative relationship between immune response triggered by sepsis, oxidative stress and their impact on dysbiosis. Dashed arrows indicate counterregulation of inflammation and oxidative stress.
Considering the findings in the latest publications, the diagnosis of neonatal sepsis should focus on the host’s response and not so much on the search for the causative germ. The differences identified in the immune response, depending on the type of infectious agent, will guide the treatment, not only earlier, but also with a shorter duration. On the other hand, metabolomics will allow researchers to go a step further toward the investigation of profiles suggestive of sepsis in non-invasively accessible biological fluids, such as urine or saliva [18].
Sepsis activates the immune and inflammatory responses that generate oxidative stress, and both generate a pro-oxidant environment in the intestine that alters the microbiota. Not only sepsis, but also the use of antibiotic therapy as a treatment, plays a relevant role in this complex mechanism.
2. Immune Response
The immune system in early life goes through rapid and radical changes. Given the malleability of the immune system in the newborn period, interventions aimed at modulating its trajectory thus have the potential to translate into considerable reductions in the infectious disease burden with short and long-lasting benefits. However, an improved understanding of the underlying molecular drivers of early life immunity is a prerequisite to optimize interventions and transform the window of early life vulnerability into one of opportunity [19].
The immune response in neonates is triggered by the innate immunity reacting to the exposure to an infectious agent. Local immune sentinel cells such as monocytes and macrophages are involved in pathogen-associated molecular patterns (PAMPs) recognition through the activation of pattern recognition receptors (PRRs), essentially Toll-like (TLRs) receptors but also other intracellular receptors that include nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and retinoic acid-inducible protein (RIG)-like receptors (RLRs) [20].
The paradigms of PAMPS are lipopolysaccharide endotoxins (LPS) of the Gram-negative bacteria surface and lipoteichoic acid (LTA) and peptidoglycan of the Gram-positive bacteria wall. The immune response initiated by PRRs yields to the production of pro-inflammatory cytokines via mitogen-activated protein kinases (MAPK) and the transcription factor nuclear factor κB (NF-κB) [21]. In an attempt to amplify the innate immune response, the production of nitric oxide (NO), leukotrienes, prostaglandins, platelet activating factor (PAF), complement, pro-inflammatory cytokines and chemokines such as interleukins (IL-1ß, IL-6, IL-8, IL-12, IL-18), interferon gamma (INF-γ) or tumor necrosis factor alpha (TNF-α) is enhanced, the vascular permeability is increased, and inflammatory cells are recruited [22][23]. Cytokines are mainly produced by activated lymphocytes and macrophages and are involved in regulating inflammation through cellular proliferation and differentiation, chemotaxis and modulation immunoglobulin secretion. However, compared to adults, septic neonates’ levels of IL-1ß, TNF-α, INF-γ and IL-12 are significantly lower due to a gestational age-related decreased production of Myeloid Differentiation Factor 88 (myd88), Interferon Regulatory Factor 5 (IRF 5) and p38 [24][25].
The production of pro-inflammatory cytokines leads to the activation of endothelial cells and overexpression of cellular adhesion molecules that promote leukocytes recruitment and diapedesis. Although cytokine concentration levels seem to be adequate, leukocyte chemotaxis is limited in neonates and probably related to an insufficient up-regulation of complement receptor and inhibition caused by bacterial products [26]. Complement is involved in opsonization; it has chemotactic and anaphylactic activities that increase leukocyte aggregation and local vascular permeability. In neonates, opsonization through complement is limited and has a direct relationship with gestational age. In addition, the low levels of complement proteins and their depressed function make preterm infants especially susceptible to infection [27]. Moreover, polymorphonuclear leukocytes (PMN) show qualitative and quantitative deficiencies compared to adults. During infection, medullar PMN storage quickly decreases, apoptosis is delayed and the ability to activate cytotoxic functions is increased. PMN deformability is reduced, leading to aggregation in the intravascular space and decreased diapedesis. Medullar storage is also depleted, and immature and dysfunctional forms are released [28].
In an attempt to control the inflammatory response, anti-inflammatory cytokines such as IL-4, IL-10, IL-11, IL-13 and transforming growth factor beta (TGF-ß) are released to suppress the activation of macrophages and production of pro-inflammatory cytokines.
These mediators, especially IL-10, released by lymphocytes Th2, lymphocytes B and macrophages in response to TNFα, block the activation of phagocytic cells and fever, modify the expression of coagulation factors, and decrease intermediate reactive species of oxygen and nitrogen and other vasoactive markers. However, an overproduction of all these biomarkers can lead to the suppression of the immune function [22].
Both the pathogen’s characteristics and the pattern of circulating cytokines determine the differentiation of T helper precursor cells (Th) toward lymphocyte Th1 or Th2. Th1 cells produce INFγ, IL-2 and TNF-β that promote cellular immunity and phagocytic activity, mostly in case of intracellular infections. Th2 cells produce IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13, triggering humoral immunity and antibodies production [29].
The effectiveness of the inflammatory response is closely related to a balanced production of cytokines. Contrarily, an excessive response could be harmful to multiple organs such as the brain, kidney, lungs, cardiovascular system, liver, bowel and microcirculation, leading to a systemic inflammatory response characteristic of an overt sepsis. The instauration of a successful treatment will rapidly return biomarkers to normal levels, organ dysfunction will be solved, and the patient’s clinical status will improve. On the contrary, ineffective treatment is characterized by the persistence of elevated biomarkers, multiorgan failure, and death [30].
In addition to the initial inflammatory response, the presence of microorganisms increases the production of innate proteins with an important immunologic function that decreases the bacterial burden by increasing the bacterial permeability depending on neutrophils. These proteins are acute phase reactants such as: collectins, lactoferrin, haptoglobin, phospholipase A2, procalcitonin, C-Reactive protein or serum amyloid A. Furthermore, sepsis promotes an increase in other serum proteins with an opsonization function such as fibronectin and natural antibodies, mostly IgM, that produce circulating B lymphocytes. However, newborn plasma shows a decreased opsonization activity and poor production of these proteins, rendering this population more susceptible to infection [31].
Transcriptomic profiling from whole blood can reveal diagnostic and prognostic gene signatures in acute inflammation. Therefore, it provides an excellent and exhaustive overview of host immune response against infection. Whole blood is a rich source of cells involved in immune response such as leukocytes, monocytes and macrophages. Studies based on genome-wide expression profiles have been successfully harnessed for the diagnosis of sepsis and patient stratification based on the severity of septic shock in the pediatric and adult population [32][33]. Moreover, transcriptomic profiling has been able to discriminate septic from non-septic preterm infants in the neonatal period [34][35]. These studies found that the most significant biological processes expressed in septic preterm infants were related to innate immune and inflammatory responses with an overexpression of NF-κB and cytokines pathways, while the T cell receptor pathway was under-expressed. The most common genes overexpressed not only in neonates but also in children and adults are involved in these networks: CD177 antigen (CD177), Matrix metalloproteinase-8 (MMP-8), Haptoglobin (HP), Lipocalin-2, Olfactomedin 4 (OLFM-4) or Carcinoembryonic antigen-related cell adhesion molecule (CEACAM-1) [34][35][36][37]. Moreover, a sepsis score based on some of these genes conducted in adult population has been validated in neonatal patients [38].
Nevertheless, the innate immune function shows some changes with postnatal age. Moreover, the innate immune development at the beginning of life compared to the response at the end of life reveals similar patterns of distinct Toll-like-receptor-mediated immune responses [39]. Septic neonates, compared to septic infants, children, and adults, showed a significantly reduced expression of genes related to TLRs, Triggering Receptor Expressed on Myeloid Cells (TREM)-1, and inducible nitric oxide synthase (iNOS) signaling pathways [40][41]. Although an overexpression of MMP8, CD177 and HP has been reported in septic neonates, fold changes are lower than those observed in children and infants.
According to these results, recent studies in VLBW with LOS based on RNA sequencing found an overexpression of genes related to innate immune response and inflammatory processes such as IFN-α/β, IFN-γ, IL-1 and IL-6 as well as pathways involved in pathogen recognition through TLR, pro-inflammatory and inhibitory cytokine signaling, immune and hematological regulation, and altered cholesterol biosynthesis metabolism [42].
In this scenario, numerous studies in recent years have aimed to describe the mechanisms of the immune response, and predictive models have been proposed [43]. Moreover, transcriptomic studies have found different responses in sepsis caused by Gram-positive compared to those caused by Gram-negative bacteria not only in adults but also in children and preterm infants [44]. Preterm infants with Gram-positive sepsis showed an overexpression of genes such as CD37, CSK and TEP1 that are related to cytokine production, cell survival, and metabolic and immunomodulating responses. This equilibrium between pro- and anti-inflammatory responses could explain the better clinical outcome for Gram-positive bacterial sepsis. Previous studies in adults found significantly higher levels of pro-inflammatory cytokines (IL-1beta, IL-6, and IL-18) in Gram-positive sepsis [45].
Moreover, studies analyzing datasets of critically ill patients reported a different signature related to signaling and recognition pathways as well as host response mediated by neutrophils and cell survival [46]. An overexpression of genes related to cellular respiration such as NADH subunits B2 and B8 and UQCRH has been revealed in Gram-negative sepsis, while Gram-positive sepsis showed an overexpression of LATS2 associated with the transition of the mitotic cell cycle [47]. The most significant discriminative genes such as SRC, TLR6, CLC2, IL1B and CD40 are related to these pathways [47].
Advances in our knowledge of the intrinsic mechanisms of the immune response will not only improve our ability to attain an early and reliable diagnosis of sepsis but will also contribute to the development of new therapeutic strategies [48][49].
Although no single biomarker is still validated to diagnose neonatal sepsis and cannot substitute the blood culture, it seems relevant to direct our efforts of knowing how the response to sepsis works to be able to use these markers as potential diagnostic tools.
According to the available data, the diagnosis of neonatal sepsis in the preterm newborn should be based on the combined use of several of the currently described biomarkers as well as infectious risk factors. Probably the best combination for early sepsis would be the use of PCR and IL-6 or IL-8 in an attempt to cover the earliest and later phases of the immune response [50]. In the case of late-onset sepsis, the decision tree incorporating inflammatory markers as PCR, PCT and IL-6 reached a diagnostic accuracy of nearly 88% [51].
At the moment, the duration of antibiotic therapy is based on fixed recommendations depending on the type of germ. However, significant efforts are being devoted to the search for markers that guide researchers toward shorter treatment regimens. A recent analysis of suspected LOS in preterm infants below 32 weeks of gestational age showed that serum IL-6 and PCT levels [52] were associated with sepsis severity and mortality risk. Therefore, some authors defend the use of PCT to guide the duration of antibiotic treatment [53][54].
This entry is adapted from the peer-reviewed paper 10.3390/children10030602
References
- Puopolo, K.M.; Benitz, W.E.; Zaoutis, T.E.; Committee on Fetus and Newborn; Committee on Infectious Diseases. Management of Neonates Born at ≤ 34 6/7 Weeks’ Gestation With Suspected or Proven Early-Onset Bacterial Sepsis. Pediatrics 2018, 142, e20182896.
- Flannery, D.D.; Puopolo, K.M. Neonatal Early-Onset Sepsis. Neoreviews 2022, 23, 756–770.
- Brumbaugh, J.E.; Bell, E.F.; Do, B.T.; Greenberg, R.G.; Stoll, B.J.; DeMauro, S.B.; Harmon, H.M.; Hintz, S.R.; Das, A.; Puopolo, K.M.; et al. Incidence of and Neurodevelopmental Outcomes After Late-Onset Meningitis Among Children Born Extremely Preterm. JAMA Netw. Open. 2022, 5, e2245826.
- Coggins, S.A.; Glaser, K. Updates in Late-Onset Sepsis: Risk Assessment, Therapy, and Outcomes. Neoreviews 2022, 23, 738–755.
- Wynn, J.L.; Wong, H.R.; Shanley, T.P.; Bizzarro, M.J.; Saiman, L.; Polin, R.A. Time for a neonatal-specific consensus definition for sepsis. Pediatr. Crit. Care Med. 2014, 15, 523–528.
- Shane, A.L.; Sánchez, P.J.; Stoll, B.J. Neonatal sepsis. Lancet 2017, 390, 1770–1780.
- Biondi, E.A.; Mischler, M.; Jerardi, K.E.; Statile, A.M.; French, J.; Evans, R.; Lee, V.; Chen, C.; Asche, C.; Ren, J.; et al. Pediatric Research in Inpatient Settings (PRIS) Network. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014, 168, 844–849.
- Straub, J.; Paula, H.; Mayr, M.; Kasper, D.; Assadian, O.; Berger, A.; Rittenschober-Böhm, J. Diagnostic accuracy of the ROCHE Septifast PCR system for the rapid detection of blood pathogens in neonatal sepsis-A prospective clinical trial. PLoS ONE 2017, 12, e0187688.
- van den Brand, M.; van den Dungen, F.A.M.; Bos, M.P.; van Weissenbruch, M.M.; van Furth, A.M.; de Lange, A.; Rubenjan, A.; Peters, R.P.H.; Savelkoul, P.H.M. Evaluation of a real-time PCR assay for detection and quantification of bacterial DNA directly in blood of preterm neonates with suspected late-onset sepsis. Crit. Care 2018, 22, 105.
- Pammi, M.; Flores, A.; Leeflang, M.; Versalovic, J. Molecular assays in the diagnosis of neonatal sepsis: A systematic review and meta-analysis. Pediatrics 2011, 128, e973–e985.
- Donà, D.; Mozzo, E.; Mardegan, V.; Trafojer, U.; Lago, P.; Salvadori, S.; Baraldi, E.; Giaquinto, C. Antibiotics Prescriptions in the Neonatal Intensive Care Unit: How to Overcome Everyday Challenges. Am. J. Perinatol. 2017, 34, 1169–1177.
- Collado, M.C.; Cernada, M.; Neu, J.; Pérez-Martínez, G.; Gormaz, M.; Vento, M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr. Res. 2015, 77, 726–731.
- Ting, J.Y.; Synnes, A.; Roberts, A.; Deshpandey, A.; Dow, K.; Yoon, E.W.; Lee, K.S.; Dobson, S.; Lee, S.K.; Shah, P.S. Association Between Antibiotic Use and Neonatal Mortality and Morbidities in Very Low-Birth-Weight Infants Without Culture-Proven Sepsis or Necrotizing Enterocolitis. JAMA Pediatr. 2016, 170, 1181–1187.
- Cernada, M.; Badía, N.; Modesto, V.; Alonso, R.; Mejías, A.; Golombek, S.; Vento, M. Cord blood interleukin-6 as a predictor of early-onset neonatal sepsis. Acta Paediatr. 2012, 101, e203–e207.
- Auriti, C.; Fiscarelli, E.; Ronchetti, M.P.; Argentieri, M.; Marrocco, G.; Quondamcarlo, A.; Seganti, G.; Bagnoli, F.; Buonocore, G.; Serra, G.; et al. Procalcitonin in detecting neonatal nosocomial sepsis. Arch. Dis. Child Fetal Neonatal. 2012, 97, F368–F370.
- Stocker, M.; Fontana, M.; El Helou, S.; Wegscheider, K.; Berger, T.M. Use of procalcitonin-guided decision-making to shorten antibiotic therapy in suspected neonatal early-onset sepsis: Prospective randomized intervention trial. Neonatology 2010, 97, 165–174.
- Ng, P.C. Diagnostic markers of infection in neonates. Arch. Dis. Child Fetal Neonatal. 2004, 89, F229–F235.
- Renwick, V.L.; Stewart, C.J. Exploring functional metabolites in preterm infants. Acta Paediatr. 2022, 111, 45–53.
- Goenka, A.; Kollmann, T.R. Development of immunity in early life. J. Infect. 2015, 71 (Suppl. 1), S112–S120.
- Dias, M.L.; O’Connor, K.M.; Dempsey, E.M.; O’Halloran, K.D.; McDonald, F.B. Targeting the Toll-like receptor pathway as a therapeutic strategy for neonatal infection. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R879–R902.
- Wynn, J.L.; Levy, O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin. Perinatol. 2010, 37, 307–337.
- Ng, P.C.; Li, K.; Wong, R.P.; Chui, K.; Wong, E.; Li, G.; Fok, T.F. Proinflammatory and anti-inflammatory cytokine responses in preterm infants with systemic infections. Arch. Dis. Child Fetal Neonatal. 2003, 88, F209–F213.
- Tang, X.D.; Ji, T.T.; Dong, J.R.; Feng, H.; Chen, F.Q.; Chen, X.; Zhao, H.Y.; Chen, D.K.; Ma, W.T. Pathogenesis and Treatment of Cytokine Storm Induced by Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 13009.
- Sadeghi, K.; Berger, A.; Langgartner, M.; Prusa, A.R.; Hayde, M.; Herkner, K.; Pollak, A.; Spittler, A.; Forster-Waldl, E. Immaturity of infection control in preterm and term newborns is associated with impaired toll-like receptor signaling. J. Infect. Dis. 2007, 195, 296–302.
- Yao, Y.M.; Osuchowski, M.F.; Wang, J.H.; Pan, Z.K. Editorial: Immune Dysfunction: An Update of New Immune Cell Subsets and Cytokines in Sepsis. Front. Immunol. 2021, 12, 822068.
- Hickey, M.J.; Kubes, P. Intravascular immunity: The host-pathogen encounter in blood vessels. Nat. Rev. Immunol. 2009, 9, 364–375.
- Drossou, V.; Kanakoudi, F.; Diamanti, E.; Tzimouli, V.; Konstantinidis, T.; Germenis, A.; Kremenopoulos, G.; Katsougiannopoulos, V. Concentrations of main serum opsonins in early infancy. Arch. Dis. Child Fetal Neonatal. 1995, 72, F172–F175.
- Koenig, J.M.; Stegner, J.J.; Schmeck, A.C.; Saxonhouse, M.A.; Kenigsberg, L.E. Neonatal neutrophils with prolonged survival exhibit enhanced inflammatory and cytotoxic responsiveness. Pediatr. Res. 2005, 57, 424–429.
- Mosmann, T.R. Cytokine secretion patterns and cross-regulation of T cell subsets. Immunol. Res. 1991, 10, 183–188.
- Sriskandan, S.; Altmann, D.M. The immunology of sepsis. J. Pathol. 2008, 214, 211–223.
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390.
- Wong, H.R.; Shanley, T.P.; Sakthivel, B.; Cvijanovich, N.; Lin, R.; Allen, G.L.; Thomas, N.J.; Doctor, A.; Kalyanaraman, M.; Tofil, N.M.; et al. Genomics of Pediatric SIRS/Septic Shock Investigators. Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol. Genom. 2007, 30, 146–155.
- Wong, H.R.; Cvijanovich, N.; Lin, R.; Allen, G.L.; Thomas, N.J.; Willson, D.F.; Freishtat, R.J.; Anas, N.; Meyer, K.; Checchia, P.A.; et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med. 2009, 7, 34.
- Cernada, M.; Serna, E.; Bauerl, C.; Collado, M.C.; Pérez-Martínez, G.; Vento, M. Genome-wide expression profiles in very low birth weight infants with neonatal sepsis. Pediatrics 2014, 133, e1203–e1211.
- Wynn, J.L.; Guthrie, S.O.; Wong, H.R.; Lahni, P.; Ungaro, R.; Lopez, M.C.; Baker, H.V.; Moldawer, L.L. Postnatal Age Is a Critical Determinant of the Neonatal Host Response to Sepsis. Mol. Med. Camb. Mass. 2015, 21, 496–504.
- Smith, C.L.; Dickinson, P.; Forster, T.; Craigon, M.; Ross, A.; Khondoker, M.R.; France, R.; Ivens, A.; Lynn, D.J.; Orme, J.; et al. Identification of a human neonatal immune-metabolic network associated with bacterial infection. Nat. Commun. 2014, 5, 4649.
- Raymond, S.L.; López, M.C.; Baker, H.V.; Larson, S.D.; Efron, P.A.; Sweeney, T.E.; Khatri, P.; Moldawer, L.L.; Wynn, J.L. Unique transcriptomic response to sepsis is observed among patients of different age groups. PLoS ONE 2017, 12, e0184159.
- Sweeney, T.E.; Wynn, J.L.; Cernada, M.; Serna, E.; Wong, H.R.; Baker, H.V.; Vento, M.; Khatri, P. Validation of the Sepsis MetaScore for Diagnosis of Neonatal Sepsis. J. Pediatr. Infect. Dis. Soc. 2018, 7, 129–135.
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate immune function by Toll-like receptors: Distinct responses in newborns and the elderly. Immunity 2012, 37, 771–783.
- Webber, R.J.; Sweet, R.M.; Webber, D.S. Circulating Microvesicle-Associated Inducible Nitric Oxide Synthase Is a Novel Therapeutic Target to Treat Sepsis: Current Status and Future Considerations. Int. J. Mol. Sci. 2021, 22, 13371.
- Vance, J.K.; Rawson, T.W.; Povroznik, J.M.; Brundage, K.M.; Robinson, C.M. Myeloid-Derived Suppressor Cells Gain Suppressive Function during Neonatal Bacterial Sepsis. Int. J. Mol. Sci. 2021, 22, 7047.
- Ng, S.; Strunk, T.; Lee, A.H.; Gill, E.E.; Falsafi, R.; Woodman, T.; Hibbert, J.; Hancock, R.E.W.; Currie, A. Whole blood transcriptional responses of very preterm infants during late-onset sepsis. PLoS ONE 2020, 15, e0233841.
- Talaei, K.; Garan, S.A.; Quintela, B.M.; Olufsen, M.S.; Cho, J.; Jahansooz, J.R.; Bhullar, P.K.; Suen, E.K.; Piszker, W.J.; Martins, N.R.B.; et al. A Mathematical Model of the Dynamics of Cytokine Expression and Human Immune Cell Activation in Response to the Pathogen Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 711153.
- Cernada, M.; Pinilla-González, A.; Kuligowski, J.; Morales, J.M.; Lorente- Pozo, S.; Piñeiro-Ramos, J.D.; Parra-Lloraca, A.; Lara-Cantón, I.; Vento, M.; Serna, E. Transcriptome profiles discriminate between Gram-positive and Gram-negative sepsis in preterm neonates. Pediatr. Res. 2022, 91, 637–645.
- Feezor, R.J.; Oberholzer, C.; Baker, H.V.; Novick, D.; Rubinstein, M.; Moldawer, L.L.; Pribble, J.; Souza, S.; Dinarello, C.A.; Ertel, W.; et al. Molecular characterization of the acute inflammatory response to infections with gram-negative versus gram-positive bacteria. Infect. Immun. 2003, 71, 5803–5813.
- Li, Z.; Zhang, Y.; Liu, Y.; Liu, Y.; Li, Y. Identification of key genes in Gram-positive and Gram-negative sepsis using stochastic perturbation. Mol. Med. Rep. 2017, 16, 3133–3146.
- Wang, Q.; Li, X.; Tang, W.; Guan, X.; Xiong, Z.; Zhu, Y.; Gong, J.; Hu, B. Differential Gene Sets Profiling in Gram-Negative and Gram-Positive Sepsis. Front. Cell. Infect. Microbiol. 2022, 12, 801232.
- Franco, J.H.; Chen, X.; Pan, Z.K. Novel Treatments Targeting the Dysregulated Cell Signaling Pathway during Sepsis. J. Cell Signal. 2021, 2, 228–234.
- Kwiatkowski, P.; Kurzawski, M.; Łopusiewicz, Ł.; Pruss, A.; Sienkiewicz, M.; Wojciechowska-Koszko, I.; Dołęgowska, B. Preliminary evaluation of selected inflammatory cytokine gene expression in lymphocytes isolated from whole human blood infected with trans-anethole-treated Staphylococcus aureus Newman strain. Lett. Appl. Microbiol. 2022, 74, 513–518.
- Cao, I.; Lippmann, N.; Thome, U.H. The Value of Perinatal Factors, Blood Biomarkers and Microbiological Colonization Screening in Predicting Neonatal Sepsis. J. Clin. Med. 2022, 11, 5837.
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288.
- Li, T.; Li, X.; Liu, X.; Zhu, Z.; Zhang, M.; Xu, Z.; Wei, Y.; Feng, Y.; Qiao, X.; Yang, J.; et al. Association of Procalcitonin to Albumin Ratio with the Presence and Severity of Sepsis in Neonates. J. Inflamm. Res. 2022, 15, 2313–2321.
- Mathur, N.B.; Behera, B. Blood Procalcitonin Levels and Duration of Antibiotics in Neonatal Sepsis. J. Trop. Pediatr. 2019, 65, 315–320.
- Fugit, R.V.; McCoury, J.B.M.; Bessesen, M.T. Procalcitonin for sepsis management: Implementation within an antimicrobial stewardship program. Am. J. Health Syst. Pharm. 2022, zxac341.
This entry is offline, you can click here to edit this entry!
