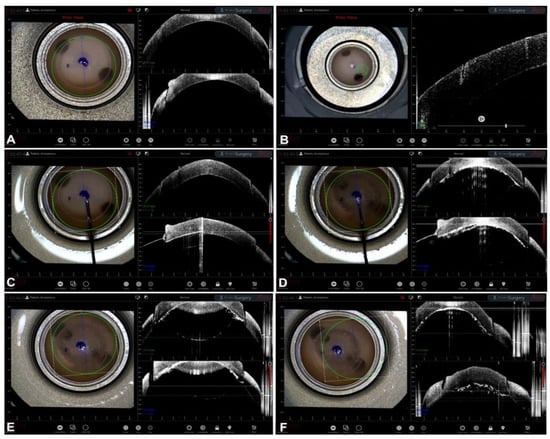
Intraoperative OCT is an innovative and promising technology which allows anterior and posterior segment ocular surgeons to obtain a near-histologic cross-sectional and tomographic image of the tissues. Intraoperative OCT has several applications in ocular surgery which are particularly interesting in the context of corneal transplantation. Indeed, iOCT images provide a direct and meticulous visualization of the anatomy, which could guide surgical decisions. In particular, during both big-bubble and manual DALK, the visualization of the relationship between the corneal layers and instruments allows the surgeon to obtain a more desirable depth of the trephination, thus achieving more type 1 bubbles, better regularity of the plane, and a reduced risk of DM perforation. During EK procedures, iOCT supplies information about proper descemetorhexis, graft orientation, and interface quality in order to optimize the postoperative adhesion and reduce the need for re-bubbling. Finally, mushroom PK, a challenging technique for many surgeons, can be aided through the use of iOCT since it guides the correct apposition of the lamellae and their centration. The technology of iOCT is still evolving: a larger field of view could allow for the visualization of all surgical fields, and automated tracking and iOCT autofocusing guarantee the continued centration of the image.
- anterior segment imaging
- multimodal imaging
- optical coherence tomography
- DALK
1. Intraoperative OCT: Technology and Characteristics
2. Intraoperative OCT Applications for Lamellar Corneal Surgery
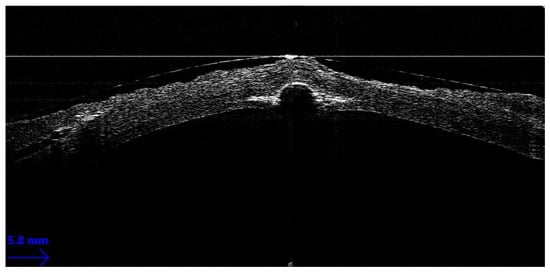
Guiding Big-Bubble Deep Anterior Lamellar Keratoplasty (BB-DALK)
This entry is adapted from the peer-reviewed paper 10.3390/jcm12093048
References
- Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Lin, C.P.; Huang, D.; Schuman, J.S.; Puliafito, C.A.; Fujimoto, J.G. Micrometer-Scale Resolution Imaging of the Anterior Eye In Vivo with Optical Coherence Tomography. Arch. Ophthalmol. 1994, 112, 1584–1589.
- Mallipatna, A.; Vinekar, A.; Jayadev, C.; Dabir, S.; Sivakumar, M.; Krishnan, N.; Mehta, P.; Berendschot, T.; Yadav, N.K. The use of handheld spectral domain optical coherence tomography in pediatric ophthalmology practice: Our experience of 975 infants and children. Indian J. Ophthalmol. 2015, 63, 586–593.
- Ang, M.; Dubis, A.M.; Wilkins, M.R. Descemet Membrane Endothelial Keratoplasty: Intraoperative and Postoperative Imaging Spectral-Domain Optical Coherence Tomography. Case Rep. Ophthalmol. Med. 2015, 2015, 506251.
- Branchini, L.A.; Gurley, K.; Duker, J.S.; Reichel, E. Use of Handheld Intraoperative Spectral-Domain Optical Coherence Tomography in a Variety of Vitreoretinal Diseases. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 49–54.
- Khademi, M.R.; Riazi-Esfahani, M.; Mazloumi, M.; Khodabandeh, A.; Riazi-Esfahani, H. Macular surgery using intraoperative spectral domain optical coherence tomography. J. Ophthalmic Vis. Res. 2015, 10, 309–315.
- Mendez, N.; Nayak, N.V.; Kolomeyer, A.M.; Szirth, B.C.; Khouri, A.S. Feasibility of Spectral Domain Optical Coherence Tomography Acquisition Using a Handheld Versus Conventional Tabletop Unit. J. Diabetes Sci. Technol. 2015, 10, 277–281.
- De Benito-Llopis, L.; Mehta, J.S.; Angunawela, R.I.; Ang, M.; Tan, D.T. Intraoperative Anterior Segment Optical Coherence Tomography: A Novel Assessment Tool during Deep Anterior Lamellar Keratoplasty. Am. J. Ophthalmol. 2013, 157, 334–341.e3.
- Ray, R.; Barañano, D.E.; Fortun, J.A.; Schwent, B.J.; Cribbs, B.E.; Bergstrom, C.S.; Hubbard, G.B.; Srivastava, S.K. Intraoperative Microscope-Mounted Spectral Domain Optical Coherence Tomography for Evaluation of Retinal Anatomy during Macular Surgery. Ophthalmology 2011, 118, 2212–2217.
- Ehlers, J.P.; Dupps, W.J.; Kaiser, P.K.; Goshe, J.; Singh, R.P.; Petkovsek, D.; Srivastava, S.K. The Prospective Intraoperative and Perioperative Ophthalmic ImagiNg with Optical CoherEncE TomogRaphy (PIONEER) Study: 2-Year Results. Am. J. Ophthalmol. 2014, 158, 999–1007.e1.
- Ehlers, J.P. Intraoperative optical coherence tomography: Past, present, and future. Eye 2015, 30, 193–201.
- Ehlers, J.P.; Modi, Y.S.; Pecen, P.E.; Goshe, J.; Dupps, W.J.; Rachitskaya, A.; Sharma, S.; Yuan, A.; Singh, R.; Kaiser, P.K.; et al. The DISCOVER Study 3-Year Results: Feasibility and Usefulness of Microscope-Integrated Intraoperative OCT during Ophthalmic Surgery. Ophthalmology 2018, 125, 1014–1027.
- Yee, P.; Sevgi, D.D.; Abraham, J.; Srivastava, S.K.; Le, T.K.; Uchida, A.; Figueiredo, N.; Rachitskaya, A.V.; Sharma, S.; Reese, J.; et al. iOCT-assisted macular hole surgery: Outcomes and utility from the DISCOVER study. Br. J. Ophthalmol. 2020, 105, 403–409.
- Wykoff, C.C.; Berrocal, A.M.; Schefler, A.C.; Uhlhorn, S.R.; Ruggeri, M.; Hess, D. Intraoperative OCT of a Full-Thickness Macular Hole Before and After Internal Limiting Membrane Peeling. Ophthalmic Surg. Lasers Imaging Retin. 2010, 41, 7–11.
- Huang, H.J.; Sevgi, D.D.; Srivastava, S.K.; Reese, J.; Ehlers, J.P. Vitreomacular Traction Surgery from the DISCOVER Study: Intraoperative OCT Utility, Ellipsoid Zone Dynamics, and Outcomes. Ophthalmic Surg. Lasers Imaging Retin. 2021, 52, 544–550.
- Falkner-Radler, C.I.; Glittenberg, C.; Gabriel, M.; Binder, S. Intrasurgical microscopeintegrated spectral domain optical coherence Tomography-Assisted membrane peeling. Retina 2015, 35, 2100–2106.
- Bruyère, E.; Philippakis, E.; Dupas, B.; Nguyen-Kim, P.; Tadayoni, R.; Couturier, A. Benefit of intraoperative optical coherence tomography for vitreomacular surgery in highly myopic eyes. Retina 2018, 38, 2035–2044.
- Ehlers, J.P.; Khan, M.; Petkovsek, D.; Stiegel, L.; Kaiser, P.K.; Singh, R.P.; Reese, J.L.; Srivastava, S.K. Outcomes of Intraoperative OCT–Assisted Epiretinal Membrane Surgery from the PIONEER Study. Ophthalmol. Retin. 2018, 2, 263–267.
- Abraham, J.R.; Srivastava, S.K.; Le, T.K.; Sharma, S.; Rachitskaya, A.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT-Assisted Retinal Detachment Repair in the DISCOVER Study: Impact and Outcomes. Ophthalmol. Retin. 2020, 4, 378–383.
- Abraham, J.; Srivastava, S.K.; Reese, J.L.; Ehlers, J.P. Intraoperative OCT Features and Postoperative Ellipsoid Mapping in Primary Macula-Involving Retinal Detachments from the PIONEER Study. Ophthalmol. Retin. 2018, 3, 252–257.
- Heindl, L.M.; Siebelmann, S.; Dietlein, T.; Hüttmann, G.; Lankenau, E.; Cursiefen, C.; Steven, P. Future Prospects: Assessment of Intraoperative Optical Coherence Tomography in Ab Interno Glaucoma Surgery. Curr. Eye Res. 2014, 40, 1288–1291.
- Zaldivar, R.; Zaldivar, R.; Adamek, P.; Cerviño, A. Intraoperative adjustment of implantable collamer lens vault by lens rotation aided by intraoperative OCT. J. Cataract. Refract. Surg. 2022, 48, 999–1003.
- Toro, M.D.; Milan, S.; Tognetto, D.; Rejdak, R.; Costagliola, C.; Zweifel, S.A.; Posarelli, C.; Figus, M.; Rejdak, M.; Avitabile, T.; et al. Intraoperative Anterior Segment Optical Coherence Tomography in the Management of Cataract Surgery: State of the Art. J. Clin. Med. 2022, 11, 3867.
- Carlà, M.M.; Boselli, F.; Giannuzzi, F.; Gambini, G.; Caporossi, T.; De Vico, U.; Mosca, L.; Guccione, L.; Baldascino, A.; Rizzo, C.; et al. An Overview of Intraoperative OCT-Assisted Lamellar Corneal Transplants: A Game Changer? Diagnostics 2022, 12, 727.
- Eguchi, H.; Hotta, F.; Kusaka, S.; Shimomura, Y. Intraoperative Optical Coherence Tomography Imaging in Corneal Surgery: A Literature Review and Proposal of Novel Applications. J. Ophthalmol. 2020, 2020, 1–10.
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.-V.; Aiello, F.; Day, A.C.; Allan, B.D. Deep Anterior Lamellar Keratoplasty for Keratoconus: Multisurgeon Results. Am. J. Ophthalmol. 2019, 201, 54–62.
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.-V.; Aiello, F.; Day, A.C.; Gore, D.M.; Allan, B.D. Femtosecond Laser–Assisted Deep Anterior Lamellar Keratoplasty for Keratoconus: Multi-surgeon Results. Am. J. Ophthalmol. 2020, 220, 191–202.
- Han, D.C.; Mehta, J.S.; Por, Y.M.; Htoon, H.M.; Tan, D.T. Comparison of Outcomes of Lamellar Keratoplasty and Penetrating Keratoplasty in Keratoconus. Am. J. Ophthalmol. 2009, 148, 744–751.e1.
- Riss, S.; Heindl, L.M.; Bachmann, B.O.; Kruse, F.E.; Cursiefen, C. Pentacam-Based Big Bubble Deep Anterior Lamellar Keratoplasty in Patients with Keratoconus. Cornea 2012, 31, 627–632.
- Santorum, P.; Yu, A.C.; Bertelli, E.; Busin, M. Microscope-Integrated Intraoperative Optical Coherence Tomography–Guided Big-Bubble Deep Anterior Lamellar Keratoplasty. Cornea 2021, 41, 125–129.
- Titiyal, J.S.; Kaur, M.; Nair, S.; Sharma, N. Intraoperative optical coherence tomography in anterior segment surgery. Surv. Ophthalmol. 2020, 66, 308–326.
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior Segment Optical Coherence Tomography–Guided Big-Bubble Technique. Ophthalmology 2013, 120, 471–476.
- Myerscough, J.; Friehmann, A.; Busin, M.; Goor, D. Successful Visualization of a Big Bubble during Deep Anterior Lamellar Keratoplasty using Intraoperative OCT. Ophthalmology 2019, 126, 1062.
- Ehlers, J.P.; Goshe, J.; Dupps, W.; Kaiser, P.; Singh, R.P.; Gans, R.; Eisengart, J.; Srivastava, S.K. Determination of Feasibility and Utility of Microscope-Integrated Optical Coherence Tomography During Ophthalmic Surgery: The DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015, 133, 1124–1132.
- Altaan, S.L.; Termote, K.; Elalfy, M.S.; Hogan, E.; Werkmeister, R.; Schmetterer, L.; Holland, S.; Dua, H.S. Optical coherence tomography characteristics of different types of big bubbles seen in deep anterior lamellar keratoplasty by the big bubble technique. Eye 2016, 30, 1509–1516.
- Steven, P.; Le Blanc, C.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huettmann, G.; Cursiefen, C. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). Br. J. Ophthalmol. 2014, 98, 900–904.
