Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Under physiological conditions, skin mast cells play an important role as guardians that quickly react to stimuli that disturb homeostasis. These cells efficiently support, fight infection, and heal the injured tissue. The substances secreted by mast cells allow for communication inside the body, including the immune, nervous, and blood systems. Pathologically non-cancerous mast cells participate in allergic processes but also may promote the development of autoinflammatory or neoplastic disease.
- mast cell activation
- urticaria
- Kounis syndrome
- Ehlers–Danlos syndrome
1. Introduction
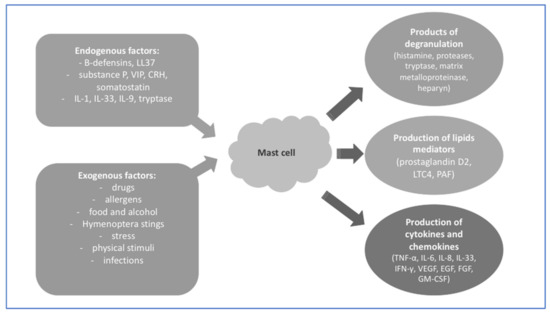
Mast cells (MCs) are mononuclear cells originating from pluripotential hematopoietic cells in the bone marrow. MCs are derived from the myeloid lineage as granulocytes, monocytes, erythrocytes, and megakaryocytes [1]. Progenitors with mast-cell-forming potential are defined as CD34+, KIT+, FcεRI + cells [2]. According to some authors, the integrin α4β7 mediates the migration of MC progenitors to peripheral tissues, where they finally differentiate into mature cells [3]. The mastocyte progenitor cell transforms into a mature MC ultimately in the peripheral tissue under the influence of various factors of a specific microenvironment. The diversification, growth, and maturing of MCs in tissues may take between several days and up to even several weeks [4].
MCs can be divided into two groups, depending on the secreted granularity: MCs(T)—MCs releasing only tryptase—and MCs(TC)—MCs secreting, in addition to tryptase, chymase, caboxypeptidase, and a cathepsin G-like proteinase. MCs(T) are founded in the mucous membranes of the respiratory system and the digestive tract, that is, in places inside of the body where MCs come into contact with factors from the outside world (e.g., food, pollen, drugs, microorganisms). However, MCs(TC) localize in the submucosa and connective tissue adjacent to the conjunctiva and skin [5][6].
Due to the strategic location of MCs in tissues adjacent to the external environment, they physiologically play a role in the processes of innate and acquired immunity against microbes, as well as stimulate tissue healing after trauma. Moreover, MCs are involved in the pathogenesis of many allergic, autoinflammatory, and cancer diseases [7].
MCs express a lot of different membrane receptors and molecules, and are capable of producing a wide range of mediators, cytokines, and chemokines, with pro- or anti-inflammatory effects.
Tryptase is a serine protease stored in basophil granules and MCs, secreted outside the cell, trigger the inflammatory cascade. Experiments in animals confirmed the role of tryptase in promoting inflammatory cell recruitment, vascular permeability, and airway hypersensitivity and remodeling [8]. Studies show that an elevated basal tryptase level (BTL), defined clinically as > 11.4 ng/mL, affects 4–6% of the general human population [9][10]. The increased BTL may be associated with mastocytosis, renal failure, and hereditary alpha tryptasemia, and may also appear as a certain individual feature without a diagnosed disease [11].
2. Mast Cells and Their Role in the Skin
The skin is the largest organ in the human body and plays a protective role against external factors such as physical, chemical, and microbiological. The presence of various cells of the immune system in the skin makes it able to effectively protect itself against microorganisms and react with healing to injury. One of the representatives of the immune system are MCs, which are involved in both innate and adaptive immune response [12].
The majority of MCs in the skin are MCs(TC) which occur in the greatest density in the superficial dermal zone and are mostly located near blood vessels and nerves, where they can react quickly [13].
One of the more well-known mechanisms of MC activation is the IgE-mediated reaction, the type I hypersensitivity reaction, which is associated with a rich symptomatology. The allergen–IgE–receptor FcεRI connection leads to the release of enzymes contained in MCs within a few minutes, which results in vascular dilatation and increased vascular permeability, which in the skin directly causes swelling, erythema, pruritus, and the formation of urticarial wheals. In the case of extensive reactions, mediators released from MCs dilate large vessels, leading to anaphylactic shock, which can be fatal [14]. This type of reaction more often occurs in patients with atopic dermatitis and urticaria.
In addition, MCs can be activated by IgG immunoglobulins through FcγRI- and FcγRIIa. MC stimulation by interferon gamma (INFγ) causes the formation of IgG receptors on the surface of the cell membrane, and accumulation of this receptors leads to cell degranulation and the generation of metabolites of arachidonic acid and the secretion of chemokines and cytokines [15]. Among the external factors activating the non-IgE-dependent pathway of MCs, there are also drugs or microbial antigens, e.g., lipopolysaccharide (LPS) or bacterial toxins [16].
Due to the fact that MCs in the skin are located close to sensory nerves, they can be endogenously stimulated by neuropeptides such as neurotensin (NT), nerve growth factor (NGF), substance P (SP), pituitary adenylate cyclase-activating polypeptide (PACAP), and vasoactive intestinal polypeptide (VIP). MCs themselves can also synthesize SP or NGF, suggesting an autocrine or paracrine mechanism [17][18][19]. High concentrations of SP cause the degranulation of MCs. SP in combination with an interleukin-1 (IL-1) family member, interleukin-33 (IL-33), increases the secretion of pro-inflammatory cytokines such as tumor necrosis factor α (TNF- α) and IL-1β [20]. VIP mediates the activation of MCs through vasoactive intestinal peptide receptor 2 (VPAC2) and/or Mas-related G-protein couplet receptor member X2 (MRGPRX2) receptors. Together, SP and VIP release cytokines and chemokines by MCs, including TNF-α, granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), C-C motif chemokine ligand 2 (CCL2), C-C motif chemokine ligand 5 (CCL5), C-X-C motif chemokine ligand 8 (CXCL8/IL-8), C-X-C motif chemokine ligand 9 (CXCL9), and C-X-C chemokine ligand 10 (CXCL10) [21].
MCs can also be activated through psychological stress. This happens through the hormones of the hypothalamus–pituitary–adrenal axis. On the surface of MCs there is mainly corticotropin-releasing hormone receptor 1 (CRH-R1). Corticotropin-releasing hormone (CRH) causes vasodilation in the skin, but the mechanism of action is not clear. On the one hand, CRH has a vasodilatory effect but also activates MCs, whose granules also have the same effect on blood vessels [22]. NT stimulates human MCs to release vascular endothelial growth factor (VEGF) and augments the effect of CRH on VEGF specific to NT, but this mechanism is not yet clear [23].
MCs are also the source of renin and constitute a unique non-renal renin–angiotensin system. Moreover, they contain angiotensin II and manifest gene expression of the renin–angiotensin system [24][25].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087021
References
- Doulatov, S.; Notta, F.; Laurenti, E.; Dick, J.E. Hematopoiesis: A Human Perspective. Cell Stem Cell 2012, 10, 120–136.
- Dahlin, J.S.; Malinovschi, A.; Öhrvik, H.; Sandelin, M.; Janson, C.; Alving, K.; Hallgren, J. Lin− CD34hi CD117int/Hi FcεRI+ Cells in Human Blood Constitute a Rare Population of Mast Cell Progenitors. Blood 2016, 127, 383–391.
- Grootens, J.; Ungerstedt, J.S.; Nilsson, G.; Dahlin, J.S. Deciphering the Differentiation Trajectory from Hematopoietic Stem Cells to Mast Cells. Blood Adv. 2018, 2, 2273–2281.
- Kounis, N.G.; Koniari, I.; Velissaris, D.; Tzanis, G.; Hahalis, G. Kounis Syndrome—Not a Single-Organ Arterial Disorder but a Multisystem and Multidisciplinary Disease. Balk. Med. J. 2019, 36, 212.
- Fong, M.; Crane, J.S. Histology, Mast Cells. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Elieh Ali Komi, D.; Wöhrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clinic. Rev. Allergy Immunol. 2020, 58, 342–365.
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The Ingenious Mast Cell: Contemporary Insights into Mast Cell Behavior and Function. Allergy 2022, 77, 83–99.
- Caughey, G.H. Mast Cell Tryptases and Chymases in Inflammation and Host Defense. Immunol. Rev. 2007, 217, 141–154.
- Gonzalez-Quintela, A.; Vizcaino, L.; Gude, F.; Rey, J.; Meijide, L.; Fernandez-Merino, C.; Linneberg, A.; Vidal, C. Factors Influencing Serum Total Tryptase Concentrations in a General Adult Population. Clin. Chem. Lab. Med. 2010, 48, 701–706.
- Fellinger, C.; Hemmer, W.; Wöhrl, S.; Sesztak-Greinecker, G.; Jarisch, R.; Wantke, F. Clinical Characteristics and Risk Profile of Patients with Elevated Baseline Serum Tryptase. Allergol. Immunopathol. 2014, 42, 544–552.
- Lyons, J.J. Hereditary Alpha Tryptasemia. Immunol. Allergy Clin. N. Am. 2018, 38, 483–495.
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811.
- Mast Cells as Regulators of Skin Inflammation and Immunity. Available online: http://www.medicaljournals.se/acta/content/html/10.2340/00015555-1197 (accessed on 13 November 2022).
- Zhao, W.; Kepley, C.L.; Morel, P.A.; Okumoto, L.M.; Fukuoka, Y.; Schwartz, L.B. FcγRIIa, Not FcγRIIb, Is Constitutively and Functionally Expressed on Skin-Derived Human Mast Cells. J. Immunol. 2006, 177, 694–701.
- Tkaczyk, C.; Okayama, Y.; Woolhiser, M.R.; Hagaman, D.D.; Gilfillan, A.M.; Metcalfe, D.D. Activation of Human Mast Cells through the High Affinity IgG Receptor. Mol. Immunol. 2002, 38, 1289–1293.
- Theoharides, T.C.; Kalogeromitros, D. The Critical Role of Mast Cells in Allergy and Inflammation. Ann. N. Y. Acad. Sci. 2006, 1088, 78–99.
- Toyoda, M.; Makino, T.; Kagoura, M.; Morohashi, M. Immunolocalization of Substance P in Human Skin Mast Cells. Arch. Dermatol. Res. 2000, 292, 418–421.
- Ødum, L.; Petersen, L.J.; Skov, P.S.; Ebskov, L.B. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Is Localized in Human Dermal Neurons and Causes Histamine Release from Skin Mast Cells. Inflamm. Res. 1998, 47, 488–492.
- Xiang, Z.; Nilsson, G. IgE Receptor-Mediated Release of Nerve Growth Factor by Mast Cells: IgE Receptor-Mediated Release of NGF. Clin. Exp. Allergy 2000, 30, 1379–1386.
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422.
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides Activate Human Mast Cell Degranulation and Chemokine Production. Immunology 2008, 123, 398–410.
- Cao, J.; Papadopoulou, N.; Kempuraj, D.; Boucher, W.S.; Sugimoto, K.; Cetrulo, C.L.; Theoharides, T.C. Human Mast Cells Express Corticotropin-Releasing Hormone (CRH) Receptors and CRH Leads to Selective Secretion of Vascular Endothelial Growth Factor. J. Immunol. 2005, 174, 7665–7675.
- Alysandratos, K.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH Interactions Augment Human Mast Cell Activation. PLoS ONE 2012, 7, e48934.
- Kounis, N.G. Kounis Syndrome (Allergic Angina and Allergic Myocardial Infarction): A Natural Paradigm? Int. J. Cardiol. 2006, 110, 7–14.
- Hara, M.; Ono, K.; Wada, H.; Sasayama, S.; Matsumori, A. Preformed Angiotensin II Is Present in Human Mast Cells.6. Cardiovasc Drugs Ther. 2004; 18, 420–415.
- Cookson, H.; Grattan, C. An Update on Mast Cell Disorders. Clin. Med. 2016, 16, 580–583.
- Patel, N.; Mohammadi, A.; Rhatigan, R. A Comparative Analysis of Mast Cell Quantification in Five Common Dermatoses: Lichen Simplex Chronicus, Psoriasis, Lichen Planus, Lupus, and Insect Bite/Allergic Contact Dermatitis/Nummular Dermatitis. ISRN Dermatol. 2012, 2012, 759630.
- Porebski, G.; Kwiecien, K.; Pawica, M.; Kwitniewski, M. Mas-Related G Protein-Coupled Receptor-X2 (MRGPRX2) in Drug Hypersensitivity Reactions. Front. Immunol. 2018, 9, 3027.
This entry is offline, you can click here to edit this entry!