Follicular thyroid carcinoma (FTC) is the second most common cancer of the thyroid gland, accounting for up to 20% of all primary malignant tumors in iodine-replete areas. The diagnostic work-up, staging, risk stratification, management, and follow-up strategies in patients who have FTC are modeled after those of papillary thyroid carcinoma (PTC), even though FTC is more aggressive. FTC has a greater propensity for haematogenous metastasis than PTC. Furthermore, FTC is a phenotypically and genotypically heterogeneous disease. The diagnosis and identification of markers of an aggressive FTC depend on the expertise and thoroughness of pathologists during histopathological analysis.
1. Introduction
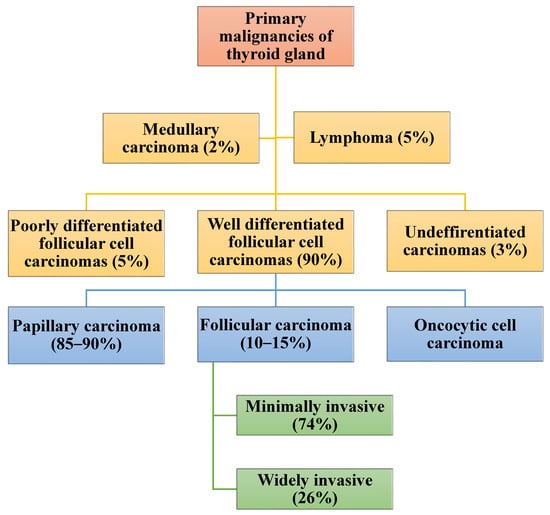
Primary thyroid carcinoma (TC) can originate from the follicular cells, para-follicular cells, or lymphoid tissues. Thyroid cancers constitute 1–5% of malignancies in adults [
1,
2]. Cancers of the thyroid of follicular cell origin are divided into well-differentiated thyroid carcinoma (WDTC), poorly differentiated and undifferentiated/anaplastic carcinoma thyroid carcinoma (ATC) [
1,
2,
3,
4,
5,
6,
7]. The WDTCs include papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), and oncocytic cell carcinoma (OC) [
8,
9]. Papillary carcinoma and FTC consist of several subtypes. The diagnosis of PTC and FTC together with their subtypes is based on the presence of classical nuclear features and the architecture of the tumor [
10,
11,
12].
Over 90% of WDTCs are sporadic. The major risk factors for WDTCs include previous exposure to ionizing radiation, a persistently abnormal level of iodine, and Hashimoto’s thyroiditis [
13,
14,
15,
16,
17]. Around 85–90% of thyroid cancers in an iodine-replete environment are classical and other subtypes of PTC [
18,
19]. The prevalence of FTC is influenced by the iodine status of that region [
20,
21,
22,
23,
24]. The experience of the histopathologists also influences the rate of diagnosis, as other benign and malignant lesions of the thyroid may be mistaken for FTC [
25]. Although FTC can occur in children, it is predominantly a disease of females over the age of 40 [
26,
27].
The majority of patients who have TC present with euthyroid goiter, and a few present due to metastasis to cervical lymph nodes or distant sites from an occult primary tumor [
28,
29]. Sometimes a WDTC is detected incidentally during the histological analysis of a specimen following a thyroidectomy for supposedly benign goiter [
30]. Sometimes, a patient who has a localized or metastatic FTC may present with hyperthyroidism [
31,
32]. The diagnostic work-up of a patient who is suspected to have TC includes thyroid function testing (TFT), ultrasound, and fine needle aspiration cytology (FNAC) [
33,
34]. The diagnosis of PTC following FNAC is based on the existence of typical nuclear features and/or architectural changes [
35]. Supplementary imaging investigations, immunohistochemistry, mutational analysis, and diagnostic thyroid lobectomy are added if FNAC is not diagnostic, which is likely in the case of FTC [
36,
37,
38,
39].
The definitive management of WDTC is either lobectomy or total thyroidectomy. Lymph node dissection, thyroid stimulating hormone (TSH) suppression, I-131 treatment, tyrosine kinase inhibitors, and external beam radiotherapy are added based on clinicopathological findings [
1,
8,
40]. The risk level of the disease influences the choice of the management strategy for WDTC [
8,
41]. Well-differentiated thyroid cancers are heterogeneous tumors with divergent clinical behavior, response to treatment, and overall outcome [
42,
43]. Risk stratification of FTC includes the age of a patient, tumour size, evidence and extent of extra-thyroidal extension. The other factors that are important in the risk stratification of a patient who has FTC are the existence lymph node or systemic metastasis, pre-operative level of thyroglobulin (Tg) and completeness of surgical excision. The histological subtype, tumour differentiation of the tumour, immunohistochemistry, genomics, epigenomics, metabolomics and the changes in the micro-environment of the tumour also have an influence on the prognosis of WDTC and therefore guide of appropriate treatment [
26,
27,
28,
41,
44,
45,
46,
47,
48,
49,
50,
51]. Additional markers that have been found to be useful in the risk stratification of patients with TC include serum Vitamin D and the neutrophil-to-lymphocyte ratio (NLR) [
52,
53].
Although observation alone or lobectomy with lifelong follow-up may be appropriate for low-risk WDTC, patients whose tumors have a high risk of local recurrence or mortality should have total thyroidectomy with or without lymph node dissection, adjuvant or therapeutic I-131, aggressive TSH suppression, and intense monitoring during follow-up [
1]. Although the 10-year survival of 90% of patients who are diagnosed with WDTC is over 95%, around 10% of the WDTCs are however unexpectedly aggressive and have a markedly reduced disease-free survival [
2,
3,
8]. Patients who have been diagnosed with WDTC need a lifelong follow-up, which should be more intense in the first year following the initiation of treatment [
1,
54,
55,
56,
57]. The follow-up program for patients who have WDTC includes clinical evaluation, neck ultrasound, monitoring of serum Tg, and radioisotope scanning, based on the patient’s risk level [
1].
Thyroid cancer is a heterogeneous disease clinically and genotypically among patients and within itself and its metastases [
58,
59]. There is also high inter- and intra-observer variability during the interpretation of the results of imaging and FNAC or histopathological specimens of follicular-patterned neoplasms of the thyroid gland [
60]. The current diagnostic and staging modalities used in WDTC are not able to accurately quantify the burden of the disease, and recurrence or progression of WDTC is sometimes detected late. Untreated WDTC has a propensity towards de-differentiating and becoming more aggressive as it progresses, and a previously low-risk and well-differentiated cancer may acquire new mutations, de-differentiate, and become aggressive and resistant to I-131 [
8,
9,
61,
62].
Follicular thyroid carcinoma cannot be diagnosed pre-operatively on FNAC because it can be confused with a follicular adenoma, rarely spread to lymph nodes, and has a different mutational landscape from that of PTC [
9,
25,
47,
63]. Additionally, FTC has a higher tendency, when compared with PTC and other thyroid malignancies, to present with systemic metastases from an occult primary tumor [
57,
64,
65]. Additionally, patients who have FTC may present with hyperthyroidism [
31,
32,
66]. The prognosis of patients with FTC is worse than that of classical PTC [
56].
Table 1 contains a summary of the comparison of FTC with PTC.
Table 1. Comparison of demography and clinicopathological features of follicular and papillary carcinomas.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11041217