Fungi can produce a large diversity of pigments. Based on their chemical structures, fungal pigments are broadly grouped into carotenoids, melanin, polyketides, and azaphilones etc. These pigments differ in many aspects, including colouration and physical and chemical properties such as molecular size, structure, hydrophobicity, reactivity and biological roles. While the same fungus may be able to produce different pigments, structurally similar pigments can be produced by fungi in evolutionarily divergent lineages. Some of these pigments play positive roles in human welfare, including vitamin precursors, antibiotics, immunomodulators and colourants. However, when present in human fungal pathogens, these pigments can also enhance the virulence and pathogenicity of these organisms. While significant progresses have been made for understanding fungal pigments, much remains unknown, including the structure-function relationships and the genes and metabolic pathways involved in their synthesis for the majority of fungal pigments.
- melanin
- carotenoids
- polyketides
- azaphilones
- antitumor
- medical roles
- fungal pathogens
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
Since prehistoric times, fungi have played critical roles in human daily activities, such as baking, brewing (wines and beers), and processing dairy products. With the advent of new technologies, especially biotechnology, over the past 60 years, the use of fungi has increased significantly for the production of commercially important products, such as edible mushrooms, drinks, alcohols, organic acids, enzymes, and colorants. After the breakthrough discovery of penicillin in the 1930s, fungi have become a very significant source of pharmaceutical products which give rise to life-saving medicines, including antibiotics, anticancer drugs, and cholesterol-lowering agents [1]. Apart from the benefits that fungi provide, some fungi can also cause significant diseases to a variety of crops, livestock, pet animals, and humans. Accumulating evidence has revealed that the incidence of human fungal diseases has been increasing rapidly since the 1980s, and is associated with excessive morbidity and mortality, particularly among immunosuppressed and immunocompromised patients [2]. Among the 625 fungal species known to be pathogenic to humans, those in the genera Aspergillus, Candida, Cryptococcus, and Trichophyton cause diseases in over 300 million people globally [2]. These diseases can be superficial, subcutaneous, or systemic. Some are acute infections, but others can be long-lasting chronic infections (e.g., athlete’s foot). Accordingly, the detailed biological processes for generating useful products from beneficial fungi and for virulence in pathogenic fungi are very active areas of research.
To the general public, when we talk about pigments in living organisms, we often think of the colorful flowers, insects, and birds. Indeed, pigments in these groups of organisms are the subjects of curiosity for people from diverse walks of life, including painters, photographers, writers, gardeners, and biologists. The biological roles for many of those pigments have been extensively studied. In contrast, unless we are in the woods and see colorful mushrooms, we often do not associate pigments with fungi, with the possible exception of “black” molds in spoiled foods. However, fungi are prolific producers of a myriad of pigments of different chemical structures and a diverse range of colors. The majority of well-studied fungal pigments are from fungi of four genera: Aspergillus, Penicillium, Paecilomyces, and Monascus [3,4]. When chemically categorized, fungal pigments are grouped into carotenoids, melanins, polyketides, azaphilones (polyketide derivatives), etc. [5,6].
Carotenoids constitute an abundant group of pigments in nature. They are found in all plants where they play an essential role in photosynthesis. In fungi, over 200 fungal species have been documented as capable of producing carotenes [7]. Carotenoids play very important roles in human health. They not only serve as the precursors of vitamin A in humans, but can also alleviate and/or prevent human age-related diseases, such as cataracts and macular degeneration. Furthermore, they can reduce the incidence of coronary heart disease and carcinomas, including lung, breast, prostate, and colorectal cancers [8]. The health-promoting effects of carotenoids are believed to be associated with their bioactivity as antioxidants and free radical scavengers.
Another broadly distributed group of fungal pigments is melanin. Melanin contributes significantly to the fungal capacity to survive and thrive in unfavorable habitats. Fungal melanin molecules are polyphenolic and/or polyindolic compounds with high molecular masses and negative charges. Due to its physiochemical properties, melanin can mediate an array of cellular functions, allowing fungal adaptation to diverse environmental factors, including ultraviolet (UV) light, heat, ionizing radiation, and oxidative stressors [9]. These properties make melanin a potential artificial stress-protecting agent (e.g., radioprotective) in bioinspired applications. However, the relevance of melanin to human health is probably best exemplified in human fungal pathogens as a virulence factor. For example, in the opportunistic human pathogens of the Cryptococcus genus, such as Cryptococcus neoformans and C. gattii, strains unable to produce melanin are avirulent or have significantly reduced virulence [10,11]. Similarly, in Aspergillus fumigatus as well as Paracoccidioides brasiliensis, the melanized cells exhibited elevated resistance to phagocytosis [12].
2. Biosynthesis of Fungal Pigments
So far, four major pathways have been identified as responsible for the biosynthesis of the main fungal pigments, including the polyketide synthetic pathway, the shikimate pathway, the terpenoid synthetic pathway, and the nitrogen-containing metabolite pathway.
2.1. Polyketide Synthetic Pathways
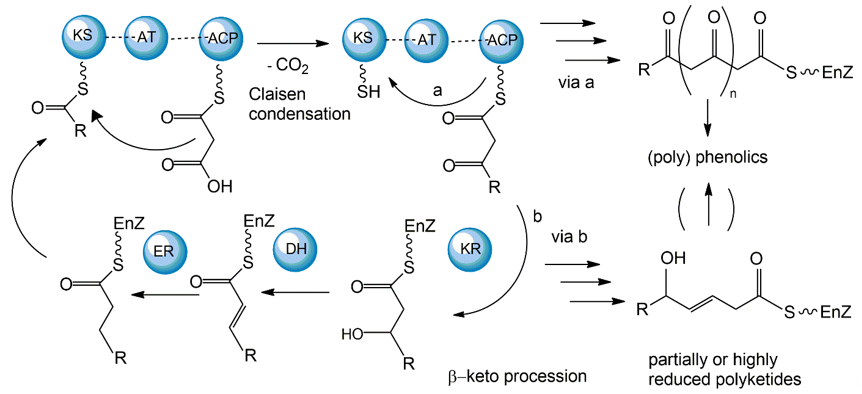
The polyketide synthetic pathway is involved in producing many fungal pigments with human health relevance. For example, there is increasing evidence that fungal pigments such as melanin, quinones, flavins, ankaflavin, and azaphilones, all relevant to human health [5,6], involve the polyketide synthesis pathway [86,87]. This pathway has been studied in diverse fungal species, including those in the genera Monascus, Fusarium, Alternaria, and Epicoccum [64]. The core polyketide synthetic pathway is shown in Figure 6. Fungal pigments are produced by this pathway via repetitive Claisen condensations of an acyl-coenzyme A (CoA) starting unit with malonyl-CoA elongation units in a manner analogous to fatty acid biosynthesis. The polyketide pathway generates either aromatic ketides or fatty acids. The extending chain of the aromatic ketides is stabilized by cyclization reactions and partial reduction. However, in the fatty acids, the carbonyl groups of the chain are reduced prior to the addition of the next C2 group. One of the major differences between the two metabolic routes is that polyketides have varied degrees of β-keto processing. Specifically, some are not or only partially reduced, giving rise to the formation of (poly-)cyclic aromatic compounds. On the other hand, they may be significantly reduced, yielding linear or macrocyclic, non-aromatic carbon skeletons.
Figure 6. Mechanisms of fungal polyketide biosynthesis.
Aromatic ketides of this pathway include tetra-, hepta-, octa- and higher number ketides. Fungi possess a variety of pigments of the octaketide origin based on the anthra-9,10-quinone backbone with both rings being substituted. Anthraquinones are representatives of this type of pigment. In many cases anthraquinones are found in fungi in the corresponding colourless reductive states (anthranol, anthrone, anthrahydroquinone, and oxanthrone derivatives) that may be present in the forms of diverse glycoside conjugants. However, many naturally occurring, coloured anthraquinones are oligomers made by the coupling of two or more anthraquinone molecules. These oligomers differ in the number and positions from which monomers and amino acids are ligated to generate a diversity of fungal pigments [88,89].
A large number of enzymes have been identified in the fungal polyketide synthesis pathway. These include a core set of enzymes, including ketosynthase (KS), acyl transferase (AT), and acyl carrier protein (ACP) domains. Further, optional β-keto processing steps may be catalyzed by keto reductase (KR), dehydratase (DH) and enoyl reductase (ER) domains. Other optional ancillary domains involve cyclase (CYC) [90] and methyl transferase (MT) activities [91]. Based on their architecture and the presence or absence of additional β-keto processing domains, fungal PKSs are categorized into non-reducing or aromatic (NR-PKS), partially reducing (PR-PKS), and highly reducing PKS (HR-PKS).
The synthesized fungal polyketides can vary in their chain length, the degree of β-keto processing, and cyclization. Moreover, the tremendous structural diversity of polyketides is further obtained from derivatization of the polyketide carbon backbone by alkylation, acylation, and oxygenation, and by post-PKS modification or tailoring. Genome sequence analyses so far have revealed that all genes essential for fungal polyketide biosynthesis are clustered, including the PKS genes, genes encoding enzymes associated with tailoring, as well as regulatory genes [92].
While most fungal polyketide pigments are synthesized as aromatic ketides, the biosynthesis of azaphilones uses both the polyketide pathway and the fatty acid synthesis pathway. The polyketide pathway assembles the main polyketide chain of the azaphilone pigments from acetic acid (the starter unit) and five malonic acid molecules (the chain extender units) in a conventional way to generate the chromophore structure. The fatty acid synthesis pathway produces a medium-chain fatty acid (octanoic or hexanoic acid) that is then bound to the chromophore by a transesterification reaction [93,94].
2.2. Shikimate Pathways
Aromatic amino acids act as the precursor of various fungal secondary metabolites such as pigments and vitamins [95]. In the fungal phylum Basidiomycota, tyrosine is the precursor of a distinct class of pigments, the betalains, solely present in the genera of Amanita and Hygrocybe [96,97]. In the phylum Ascomycota, tyrosine-derived pigments, such as melanin and tyrosine betaine, are thought to contribute to stress tolerance (e.g., temperature, radiation) [98,99] and pathogenicity [100,101].
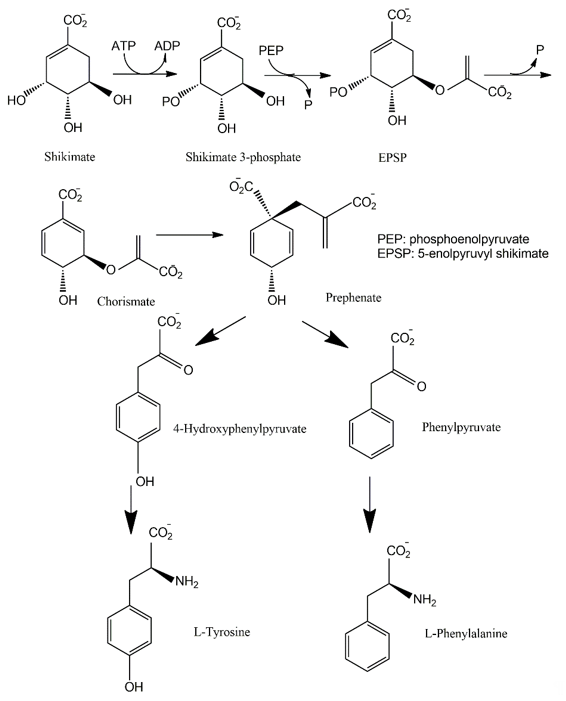
The shikimate pathway is present in the prokaryotes, microbial eukaryotes and plants studied. However, it is absent in those animal species investigated [102]. This pathway links carbohydrate metabolism to the biosynthesis of aromatic compounds. Metabolically, the shikimate pathway is a seven-step route utilized by fungi for the biosynthesis of aromatic amino acids like phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp), as well as para-aminobenzoic acid, via the central intermediates shikimic and chorismic acids [86]. The first step involves the condensation of the glycolytic intermediate phosphoenol pyruvate and pentose phosphate pathway intermediate erythrose-4-phosphate to yield a seven-carbon heterocyclic compound, 3-deoxy-D-arabinose-heptulosonate-7-phosphate derivative (DAHP). The second step involves the generation of a highly substituted cyclohexane derivative, 3-dehydroquinate, by the replacement of the exocyclic C7 of DAHP by the ring oxygen. The remaining five steps involve the introduction of a side chain and two of the three double bonds that convert this cyclohexane into a benzene ring, the core of aromatic amino acids [5]. The metabolic routes may vary for different classes of pigments.
The pigments produced via the shikimate pathway are generally water-soluble phenolic compounds, including terphenyls and pulvinic acids. Studies of p-terphenyls as a family of the mushroom pigments were initiated in 1877. Isolation of polyporic acid, atromentin and thelephoric acid represented the inception of the chemical investigation of fungal pigments. The elucidation of the structures of polyporic acid and atromentin by Kὅgl is recognized as a milestone in organic chemistry [103]. It has been demonstrated that some terphenyls display biological activities, such as immunosuppressive, antithrombotic, anticoagulant, neuroprotective, 5-lipoxygenase inhibitory (for the treatment of inflammatory bowel disease), and cytotoxic activities [104].
Many fungi have evolved pathways utilizing the aromatic products of shikimate metabolism (Figure 7). The initial steps in the biogenesis of p-terphenyls are the well-known reactions of primary metabolism flowing from shikimate to chorismic acids and subsequently to arylpyruvic acids. Experiments involving the feeding of 13C- and 14C-labeled precursors to fungal cultures revealed that p-terphenyls are assembled by initial condensation between two molecules of unbranched phenylpropanoid precursors, either phenylpyruvic acid or phenylalanine. Previous studies also revealed the involvement of 4-hydroxyphenylpyruvic acids or tyrosine in the initial condensation during the biosynthesis of terphenylquinones such as atromentin [105]. Atromentin is known as a key intermediate for further conversions, for instance, to more highly hydroxylated terphenylquinones and pulvinic acids [106,107].
Figure 7. Shikimate pathway leading to biosynthesis of p-terphenyls.
2.3. Terpenoid Synthetic Pathways
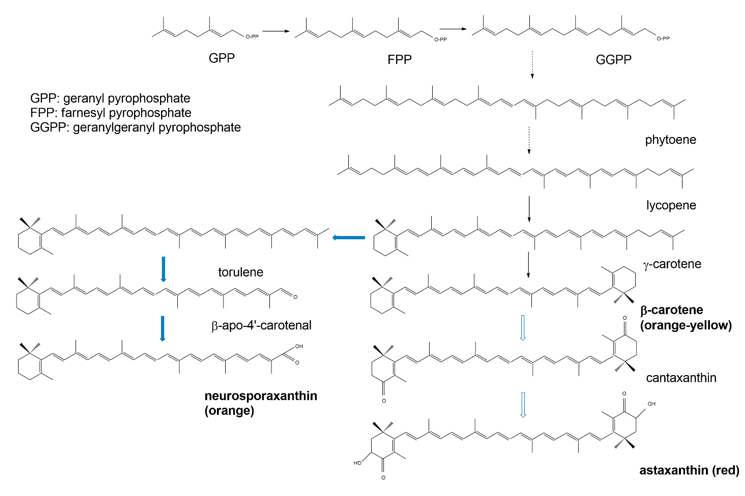
Biochemically, the carotenoids are terpenoids [15]. Accordingly, the synthesis of carotenoids utilizes the terpenoid synthetic pathways deriving from the condensation of C5 isoprene units [108]. The common terpenoid precursor is isopentenyl pyrophosphate (IPP), which is synthesized either via the mevalonate pathway (generated from hydroxymethylglutaryl coenzyme A, HMG-CoA), or via the non-mevalonate pathway (generated from the condensation of pyruvate and glyceraldehyde 3-phosphate [109]. So far, available research has demonstrated that fungal IPP is produced via the mevalonate pathway, whereas in bacteria and photosynthetic species IPP is produced via the non-mevalonate pathway. The early biosynthetic steps involve the sequential additions of IPP (isoprene, C5) units to yield geranyl pyrophosphate (GPP, C10), farnesyl pyrophosphate (FPP, C15), and geranylgeranyl pyrophosphate (GGPP, C20) [108,109]. The initial compound possessing the typical aliphatic carotenoid-like structure is 15-cis-phytoene (a colorless molecule), consisting of a symmetrical polyene chain generated via the condensation of two GGPP units catalyzed by phytoene synthase (Figure 8). The light-absorbing characteristics of carotenoids are attributed to the presence of a chromophore, which is comprised of an array of conjugated double bonds via the catalysis of desaturases. Different subsequent chemical modifications, usually the introduction of a cyclic end group (with β and ε rings being the most frequently introduced) into at least one of the ends of the molecule, and/or oxidative reactions (for instance, hydroxylation, carboxylation, epoxidation, esterification, etc.), may contribute to the huge arsenal of carotenoids [108].
Figure 8. Simplified scheme of the biosynthetic pathways for β-carotene (black arrows) and the astaxanthin and neurosporaxanthin (blue arrows) from GPP.
2.4. Nitrogen-Containing Metabolite Pathways
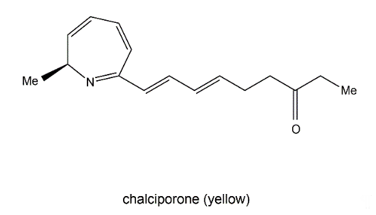
Chalciporone (Figure 9) is a type of 2H-azepine alkaloid pigment produced by the mushroom Chalciporus piperatus (Basidiomycetes). This pigment is believed to act as a deterrent to insects and other predators and thereby can potentially protect the mushroom. The biosynthesis of chalciporone was illustrated in 2001 [110]. In their experimentations, the radioactive labelled sodium [U-13C2] acetate was not incorporated into chalciporone by C. piperatus. In contrast, the supplementation of a mixture of [U-13C]-labelled fats led to chalciporone, in which seven intact acetate units were incorporated (ca. 10 atom % enrichment) between C3 and C16. However, the enrichment of the C1 methyl group and the adjacent carbon (C2) was not observed during this experiment, suggesting that the CH3–CH–N moiety was derived from an α-amino acid. This was confirmed by the observation that administration of a mixture of [U-13C]-labelled amino acids to C. piperatus gave rise to the enrichment of both C1 and C2 and of all the other carbon signals in the spectrum of chalciporone [107]. The work clearly demonstrated that the carbon backbone of chalciporone is generated from an amino acid plus seven acetate (= malonate) units.
Figure 9. Chalciporone.
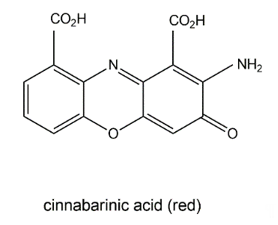
The wood-rotting fungus Pycnoporus cinnabarinus (Basidiomycetes) can produce a red pigment, cinnabarinic acid (Figure 10), via oxidative dimerization of the precursor 3-hydroxyanthranilic acid in sporocarps as well as in culture broth [111]. This reaction is catalyzed by laccase and necessary for the production of antibacterial compounds by the fungus. Cinnabarinic acid shows inhibitory effects towards several Gram-positive bacteria of the Streptococcus genus. It is known that cinnabarinic acid shares structural homology with the antibiotic group of actinomycins (e.g., actinomycin D) produced by Streptomyces spp., having two cyclic pentapeptides linked to the phenoxazinone chromophore.
Figure 10. Cinnabarinic acid.
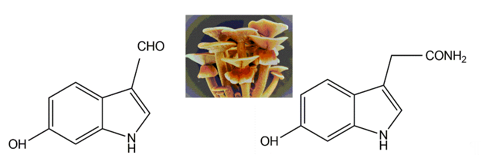
Two indole pigments were isolated as free radical scavengers from the fruiting bodies of the mushroom Agrocybe cylindracea [112]. Based on spectroscopic data, they were identified as 6-hydroxy-1H-indole-3-carboxaldehyde and 6-hydroxy-1H-indole-3-acetamide (Figure 11). 6-hydroxy-1H-indole-3-acetamide is an amide derivative of 6-hydroxyindole-3-acetic acid. A previous study identified that 6-hydroxyindole-3-acetic acid could be produced through the transformation of indole-3-acetic acid by Aspergillus niger [113]. These two indolic compounds were found to possess potent inhibitory activity on lipid peroxidation in rat liver microsomes, with IC50 values of 4.1 and 3.9 μg per ml, respectively [109].
Figure 11. The indolic compounds 6-hydroxy-1H-indole-3-carboxaldehyde (left) and 6-hydroxy-1H-indole-3-acetamide (right) isolated from the fruiting bodies of Agrocybe cylindracea (middle, insert image).
References
- Demain, A.L.; Vandamme, E.J.; Collins, J.; BuchhoIz. History of industrial biotechnology. In Industrial Biotechnology: Microorganisms; Wittmann, C., Liao, J.C., Eds.; Wiley-VCH: Weinheim, Germany, 2017; pp. 3–84.
- Boral, H.; Metinb, B.; Döğenc, A.; Seyedmousavi, S.; Ilkit, M. Overview of selected virulence attributes in Aspergillus fumigatus, Candida albicans, Cryptococcus neoformans, Trichophyton rubrum and Exophiala dermatitidis. Fungal Genet. Biol. 2018, 111, 92–107.
- Gunasekaran, S.; Poorniammal, R. Optimization of fermentation conditions for red pigment production from Penicillium under submerged cultivation. Afr. J. Biotechnol. 2008, 7, 1894–1898.
- Méndez, A.; Pérez, C.; Monta˜éz J.C.; Martínez, G.; Aguilar, C.N. Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. Zhejiang Univ. Sci. B (Biomed. Biotechnol.) 2011, 12, 961–968.
- Akilandeswari1, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Microbiol. Biotechnol. 2016, 100, 1631–1643.
- Dos Reis Celestino, J.; De Carvalho, L.E.; Da Paz Lima, M.; Lima, A.M.; Ogusku, M.M.; De Souza, J.V.B. Bioprospecting of amazon soil fungi with the potential for pigment production. Process Biochem. 2014, 49, 569–575.
- Dufosse, ; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N. C.; Ravishanka, G.A. Microorganisms and microalgae as source of pigments for use: A scientific oddity or an industrial reality? Trends. Food Sci. Technol. 2005, 16, 389–406.
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Lipid Res. 2004, 43, 228–265.
- Lee, D.; Jang, E.-H.; Lee, M.; Kim, S.-W.; Lee, Y.; Lee, K.-T.; Bahn, Y-S. Unraveling melanin biosynthesis and signaling networks in Cryptococcus neoformans. mBio 2019, 10, e02267-19.
- Chang, P.-K.; Cary, J.W.; Lebar, M.D. Biosynthesis of conidial and sclerotial pigments in Aspergillus Appl. Microbiol. Biotech. 2020, 104, 2277–2286.
- Brilhantea, R.S.N.; da Rochab, M.G.; de Oliveiraa, J.S.; Pereira-Neto, W.A.; de Melo Guedes, G.M.; de Aguiar Cordeiro, R.; Sidrim, J.J.; Rocha, M.F.; Castelo, D.D. Cryptococcus neoformans/Cryptococcus gattii species complex melanized by epinephrine: Increased yeast survival after amphotericin B exposure. Pathog. 2020, 143, 104123.
- Santos, L.A.; Grisolia, J.C.; Burger, E.; de Araujo Paula, F.B.; Dias, A.L.T.; Malaquias, L.C.C. Virulence factors of Paracoccidioides brasiliensis as therapeutic targets: A review. Antonie Leeuwenhoek J. Gen. 2020, 113, 593–604.
This entry is adapted from the peer-reviewed paper 10.3390/jof6040280