Radiopharmaceutical therapy, which can detect and treat tumours simultaneously, was introduced more than 80 years ago, and it has changed medical strategies with respect to cancer. Many radioactive radionuclides have been developed, and functional, molecularly modified radiolabelled peptides have been used to produce biomolecules and therapeutics that are vastly utilised in the field of radio medicine. Advanced technologies, such as conjugation of functional peptides or incorporation of radionuclides into chelating ligands, have been developed for advanced radiopharmaceutical cancer therapy. New radiolabelled conjugates for targeted radiotherapy have been designed to deliver radiation directly to cancer cells with improved specificity and minimal damage to the surrounding normal tissue. The development of new theragnostic radionuclides, which can be used for both imaging and therapy purposes, allows for more precise targeting and monitoring of the treatment response. The increased use of peptide receptor radionuclide therapy (PRRT) is also important in the targeting of specific receptors which are overexpressed in cancer cells.
1. Introduction
Because cancer is one of the diseases with the highest mortality rates, the battle against cancer has attracted the attention of many scientists. Researchers have investigated a wide variety of approaches to treat malignant tumours, including photothermal, photodynamic, sonodynamic, and immune therapy [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Despite the significant developments made in the fight against cancer in recent years, no single therapy has been successful in treating the disease entirely. Better patient selection, prediction of treatment response and tissue toxicity, and response evaluation are all possible through theragnostics, since diagnostic and therapeutic methods relating to the same precise molecular targets can be coupled. Although the term theragnostics was originally put forth back in 2002, the notion underlying theragnostics has been used and explored over the years [
12]. Nuclear theragnostics involves the use of radioactive compounds for imaging biological phenomena by detecting the expression of disease-related targets such as cell surface receptors or membrane transporters, followed by the application of agents designed to deliver ionising radiation to the tissues that express these targets. For the purpose of detection, a positron-emitting nuclide is injected intravenously, and the number of positrons emitted in the body is monitored using a detection camera. Images of nuclide distribution provide insight into the illness. The nuclides vary in their physical characteristics [
13]. For effective diagnosis and therapy, it is preferable to employ the nuclides most suited to the particular disease in question. This is why it is important to think about aspects such as the linker, dosage, medicinal drug to be conjugated with, etc., as well as the nuclides themselves. The same mechanism of action underlies all forms of nuclear medicine therapy in which radiopharmaceutical substances are employed to selectively target cancerous tissue, and radiation is conveyed to individual cells via chemical and/or biological adherence.
There are two main types of particle radiation that are used for medical purposes, namely, radiation with α and β particles, and both of them have a similar feature: destruction of malignant cells as a result of severe DNA detrition. Some isotopes (e.g.,
131I,
186Re,
153Sm etc.) can emit both therapeutic β
− particles as well as γ rays, making simultaneous diagnosis and therapy possible [
14,
15,
16,
17,
18,
19]. Diagnostic and therapeutic radiopharmaceuticals are referred to as theragnostic pairs when they access the same cellular structure and biological process.
Radionuclides (radioactive nuclides or radioisotopes) have been studied for the treatment and diagnosis of disease for over 100 years. However, the utilisation of radionuclides in pharmaceutical medicine has faced several problems and limitations, such as the insufficiency of their targeting mechanisms. Iodine-131 is a radionuclide that was used to treat thyroid cancer in 1946, which was a great breakthrough in pharmaceutical medicine [
23]. Other attempts have been made to develop radionuclides, such as iodine-131, but they were not very successful. Later, advancements in pharmaceutical medicine improved the use of radionuclides. The newly developed selective targeting delivery of radionuclides can prevent the occurrence of unfavourable delivery, thus reducing their side effects [
24]. Moreover, the specific targeting delivery of radionuclides enhances the imaging quality at sites of interest, such as that of the tumour [
25,
26,
27,
28,
29,
30,
31]. Targeting moieties such as small molecules, proteins, peptides, and antibodies are commonly utilised for their pharmaceutical applications. Along with them, the use of artificial single-strand oligonucleotide sequences, also known as aptamers (e.g., DNA, RNA, etc.), recently gained significant attention due to their high affinity and specificity when binding with biological molecules.
As a diagnosis tool, iodine and lutetium radioisotopes co-emit a γ photon, which can be detected by single-photon emission computed tomography (SPECT) [
35]. In addition, positron emission tomography (PET) is capable of detecting β
+ decay with the emission of a positron. Further, radioisotopes can be explored as potential therapeutic agents because they have properties that enable them to emit α
− particles, β
− particles, and Auger electrons, which can damage deoxyribonucleic acid (DNA) and lead to cell apoptosis [
36,
37]. Among commonly used radioisotopes in biomedical applications are the short-lived β+ emitters
15O,
13N,
11C, and
18F, and the long-lived β
+ emitters
89Zr,
64Cu, and
52Mn [
38]. In the case of α-emitters,
213Bi,
223Ra,
211At, and
225Ac have been investigated for the treatment of cancer metastases due to their apoptotic effects [
39]. Various theragnostic strategies involving them have been studied, and some radioisotopes, such as
225Ac, have been utilised for cancer therapy in preclinical and clinical trials [
40,
41,
42,
43].
2. Various Radionuclides for Theragnosis
Diagnostic biomarkers are often coupled with therapeutic medicines because they share common targets in cancer cells or tissues. Radioactive tracers or radionuclides are the pillars of this theragnostic concept, which is also employed in precision oncology. Nuclear theragnostic agents can deliver ionization and radiation to affect tumour cells or tissue, allowing for the imaging of the targets with the use of radioactive substances. In general, radiation with α and β particles is used in nuclear medicine, as both can cause severe damage to the cells due to their ability to destroy DNA. Another class of radiotherapy uses Auger electrons, which are electrons with very low energy emitted by radionuclides. The energy that is placed in nanometre–micrometre reserves induces high linear energy transfer (LET). For this reason, when it is discharged in a close propinquity to cancer cells, it can cause immense impairment by attacking DNA, both unswervingly and meanderingly through water radiolysis [
47]. These radioisotopes, which are used in medicines, are studied thoroughly before being incorporated into clinical trials.
Holmium-166 (
166Ho)-labelled microspheres with high activity are utilised in interventional radioembolization, along with SPECT (single-photon emission computed tomography) imaging, for dosimetry purposes. The ensuing high count rate may have an impact on dead time, lowering the dosimetric precision and image quality [
49]. High purity
166Ho radioisotopes can be synthesised by one neutron activation of the
165Ho isotope. The
166Ho isotope emits two very high-energy β particles (1774.32 keV, yield 48.8%; 1854.9 keV, yield 49.9%) and γ rays (80.57 keV, yield 6.7%; 1379.40 keV, yield 0.9%). Due to its high energy, it can be visualised using a gamma camera when injected into the body [
50].
166Ho is one of the most promising radionuclides for theragnostic use due to its relatively high specific activity and short physical half-life.
Furthermore,
166Ho can be applied in theragnosis by combining it with various polymers. A relatively well-established study by Ha et al. focused on the
166Ho-chitosan complex and evaluated its theragnostic effect in 22 cystic brain tumour patients [
56].
Lutetium-177 (
177Lu) is a special nuclide that has gamma and beta co-emitting properties [
58]. This particular property is what makes
177Lu a promising cancer theragnostic. The emission of gamma rays by
177Lu allows for a visualization of the drug’s distribution in the body due to its low energy [
59]; this is called scintillation. As a moderate energy beta emitter,
177Lu has been reported to be more effective in treating small tumours than other nuclides (especially Yttrium-90, which is a stronger energy beta emitter) [
60]. One of the important factors in applying nuclides to patients is the half-life. When the half-life is too long, the nuclide stays in the patient’s body for a long time, increasing the time required for hospitalization and treatment [
61].
Samarium-153 (
153Sm) is one of the nuclides that produces high radio-nuclidic purity through the neutron bombardment of isotopically enriched
152Sm
2O
3 [
62].
153Sm decays into a stable daughter nuclide, which is
153Eu, which has a half-life of 1.9 days [
62].
153Sm also has co-emitting properties with beta particles (E
max = 705 keV, 635 keV) and gamma photons (103 keV), which are suitable not only for therapy but also for SPECT imaging [
63].
153Sm-EDTMP (Quadramet
®, Lantheus Medical Imaging, Inc., Billerica, MA, USA) has been approved by the US FDA as an excellent radionuclide for bone metastasis.
Yttrium-90 (
90Y) is one of the most clinically used nuclides due to its unique properties.
90Y emits beta particles with 0.937 MeV in energy, and the emitted beta particles can penetrate approximately 2.5 mm of tissue [
65]. It is a nuclide suitable for use in transarterial radioembolization (TARE) because it emits relatively high energy and does not penetrate deeply into tissues, resulting in a low risk of side effects. In addition,
90Y has a short half-life (2.675 days) [
66]. Due to these properties, it has been widely studied and used as a cancer theragnostic, especially for liver cancer [
67].
3. Radiopharmaceuticals with Radiolabelled Peptides towards Cancer Therapy: Mechanistic Pathway and Biological Paraphernalia
The neutralization of cancer cells by radiolabelled peptides follows the radiation-induced killing pathway in therapy. Radiopharmaceuticals are one of the most important tools for fighting cancer, and the development of peptide receptor radionuclide therapy (PRRT) depends on the knowledge gathered from radiotherapy [
90]. The mechanistic pathways of radiopharmaceuticals show that some important parameters need to be addressed, such as the complete measurement of physiological and biological functions at the targeted sites [
48]. PRRT solely depends on the process of amassing radiopharmaceuticals at the definite site, which is regulated by several biological processes such as the transference of biochemical species and enzymatic exchanges.
3.1. Radiation Dosimetry
In radiotherapy, dosimetry plays a crucial role as it reports the biological data regarding the dose absorbed by both the tumour and the normal tissue. Patient-specific dosimetry depends on the time-ordered distribution of radiopharmaceuticals, and imaging techniques are used for internal dosimetry evaluation. When the radionuclide sits on the surface of cancer cells, a small amount of released energy is deposited into the target cells [
91]. This is somewhat countered by the higher concentration that may be achieved in tiny clusters of cells as compared to large, quantifiable tumours. Different techniques can be used to obtain data on the distribution of radiopharmaceuticals in a patient, which include single-photon emission computed tomography (SPECT) and positron emission tomography (PET) [
92], as well as whole-body emission counting [
93] and planar γ-imaging [
94].
Monte Carlo (MC) simulation is a statistical method that determines the three-dimensional interactions of radioactive particles that involve a random pathway [
95]. Considering tissue penetration depth, energy loss, bremsstrahlung photons, and cross-fire dosage, the MC model is typically detailed. The key benefits of MC simulations include their capacity to take into consideration an inhomogeneous radioactivity distribution, patient-specific organ geometries, induction of secondary particles (typically γ-radiation), and transitions between tissue types [
96]. From measurement-based calculations to pencil beam algorithms to superposition/convolution algorithms, the dose calculation engines used for treatment planning in radiotherapy have continuously improved in terms of accuracy. Monte Carlo treatment planning (MCTP) was first developed in the 1990s, after the successful implementation of MC codes to derive patient-specific dose distribution data, but the main obstacle to efficacious clinical enactment has always been computer power [
97].
The Society for Nuclear Medicine’s Medical Internal Radiation Dose (MIRD) committee devised a way to calculate the average radiation doses received by patients through radiopharmaceuticals. When utilising S values, as specified in MIRD brochures no. 5 and no. 11, it is presumed that radioactivity is distributed uniformly within organs, and that organ mass is standardised [
99]. In the past, dosimetry analysis has relied on straightforward mathematical humanoid models that assume the presence of infinite homogeneous fluids with soft tissue density and include spheres of various volumes. The most recent voxel-based anthropomorphic phantoms from the MIRD/ICRP (International Commission on Radiological Protection) are designed for men, women, and children of various ages. Although diagnostic imaging may be used to create patient-specific organ masses, it is currently not possible to modify the location, tissue inhomogeneity, or form of the organs [
100,
101].
3.2. Localization Pathways
The term “passive diffusion” is used to describe the random movement of molecules from areas of greater to lower concentration in order to achieve homogeneity. However, in a living system, this kind of motion often involves molecular transport across a membrane. Molecule mobility across membranes is affected by factors such as pH, ionization, molecule size, and lipid solubility [
110]. Since phospholipids, glycolipids, sphingolipids, and sterols are the prevalent types of lipids that make up membranes—of which phospholipids are the main component—lipid solubility is the main decisive factor. As a result, only molecules that are soluble in lipids (known as lipophilic molecules) can pierce through membranes; polar hydrophilic ones cannot. The membranes prevent the diffusion of lipophobic molecules while permitting the passage of lipophilic ones. This barrier can be broken as a result of some physiological disorders, which then allow hydrophilic molecules to diffuse into the brain tissues.
99mTc-DTPA usually follows this kind of localization mechanism when used for brain imaging. Due to its hydrophilic nature, it cannot usually cross the barrier, but when abnormalities arise which create a perturbation across the blood–brain barrier (BBB), using this passive diffusion process
99mTc-DTPA crosses the barrier [
111,
112,
113,
114].
Compartmentalised localisation is the process by which one or more preferred species are disseminated inside a limited area. In a radiopharmaceutical context, “compartment-localisation” refers to the process of confining a radiotracer within a defined volume and maintaining it long enough to allow for a thorough examination of the volume. Under normal conditions, the fluids in the body’s compartments flow in a systematic pattern, but pathologic changes can disrupt this flow, producing abnormalities. In human anatomy, the vascular system, cerebrospinal fluid space, peritoneal cavity, etc., are designated as biological compartments.
111In-DTPA imaging is used to monitor the leakage of CSF [
117], which is attributed to the mechanistic pathway of unexpected leaking from its designated repository caused by pathologic alterations.
4. Fabrication of Amino Chains for Radiopharmaceutical Applications
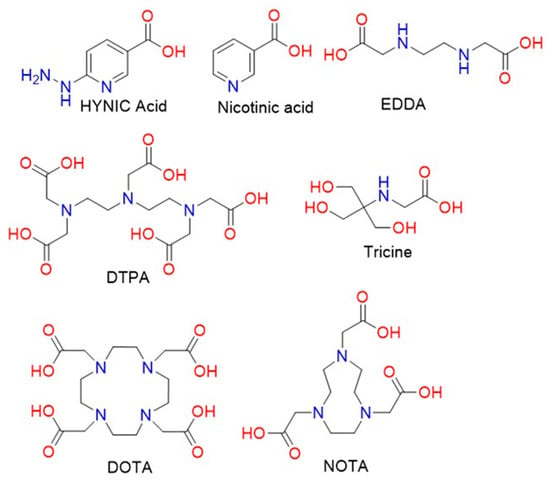
There is an increasing need to develop advanced and sophisticated procedures for successful delivery to target sites. With advances in chemistry, chelation has been chosen as a potential method for the insertion of metallic cations (e.g.,
177Lu
+3,
90Y
+3, etc.), considering the rate of metal complex formation and dissociation. The size of the cavity of bifunctional chelating agents (BFCAs) and the ionic radius of the cationic metal should be compatible in terms of metal binding with high stability and limiting dissociation [
123]. Additionally, to circumvent the possible interference between the active site of the chelator and the receptor-binding site, a linker may be required [
124]. Commonly used linkers such as PEG and amino chains have been utilised as pharmacokinetic modifiers. Many well-designed BFCAs have been explored for the fabrication of radiolabelled peptides. The common structures of acyclic and cyclic BFCAs for the development of radiolabelled amino chains are shown in
Figure 3 [
125].
Figure 3. Structural formulae of different chelators and co-ligands for the radiolabelling of peptides and proteins.
5. Radiolabelled Peptides Used in Cancer Theragnosis
5.1. Somatostatin Receptor-Targeted Anticancer Therapy
Somatostatin, a cyclic peptide, is a significant physiological modulator of neuroendocrine activity in various organ systems across the human anatomy. Its activity is mediated by five distinct SST receptor subtypes (SSTR1–5), and it inhibits the release of hormones as well as the growth of tumours. SSTR subtype expression fluctuates amongst various pituitary adenomas and tumours that secrete the same hormone [
129]. Neuroendocrine tumours (NETs) are a category of malignancies that develop from neuroendocrine cells that are widely spread throughout the body and share similar characteristics with both endocrine (hormone-producing) and nerve cells (neurons) [
130]. Numerous neuroendocrine cells, such as anterior pituitary somatotroph, thyroid C, and pancreatic islet cells, have been shown to express somatostatin receptors [
131,
132]. Among them, SST-2R is typically overexpressed in NETs, thus allowing for the development of theragnostics that selectively target SST-2R-positive NETs. This was the crucial piece of information that facilitated the first successful imaging of NETs, which was carried out by E.P. Krenning and colleagues [
133]. They developed the first radiolabelled somatostatin analogue, namely,
123I-labelled Tyr
3-octreotide. By 1990, it had been used on hundreds of patients in clinical trials, producing fine single-photon emission computed tomography (SPECT) images of localised carcinoid tumours, paragangliomas, and pancreatic endocrine tumours. However, the high biliary excretion of
123I resulted in a concentrated colonic accrual, which disrupted the construal of planar and SPECT images of tumours in the abdomen. This paved the way for the discovery of diethylenetriaminepentaacetic acid-d-phenylalanine (DTPA)-octreotide radiolabelled with indium-111 (
111In-DTPA-D-Phe
1-octreotide) [
134], which showed high sensitivity towards the localisation of NETs.
Another commercially available SSTR analogue named
99mTc-depreotide (commercial name: NeoTect), which binds with SSTR subtypes 2, 3, and 5, is used to treat patients with non-Hodgkin’s lymphoma. Following the path of Octeroscan ™, this functions in the same way by identifying the lymphoma sites. Due to its in vivo half-life, optimal biodistribution, and high binding affinity, Depreotide (cyclo-[(N-Me)Phe-Tyr-D-Trp-Lys-Val-Hcy]CH
2-CO.β-Dap-Lys-Cys-Lys.amide, P829) labelled with a beta emitter was developed as a tumour-imaging radiopharmaceutical by D.L. Bushnell and colleagues [
138].
Among other somatostatin analogues,
177Lu-DOTATATE is one of the most widely used PRRTs globally. Surprisingly, when a dosimetric study was performed by introducing an albumin-binding moiety, Evans’ Blue,
177Lu-DOTATATE showed remarkably higher uptake and retention in NETs [
140]. Based on the pharmacokinetic profile, it was evident that 2 h after the injection of
177Lu-DOTA-EB-TATE (
177Lu-1, 4, 7, 10-tetra-azacyclododecane-1, 4, 7, 10-tetraacetic acid-Evans blue-octreotate), there was high accumulation in the blood as well as a moderate uptake in the liver, spleen, and kidneys; however, in the case of
177Lu-DOTATATE, there was surprisingly no blood accumulation detected [
141].
In addition, an important study published by Thomas L. Andersen and colleagues compared [55Co]Co-DOTATATE, [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE to in vivo imaging characteristics in an SSTR-positive xenograft mouse model. The capacity of PET imaging to improve picture contrast and, hence, the detectability of cancers is dependent not only on the discovery of new targeting mechanisms with new tracers, but also on the imaging properties of the radionuclide.
5.2. CD13-Targeted Anticancer Therapy
Neo-angiogenesis in the tumour stroma recruits new blood vessels from the prior vasculature. This is a multi-step process that involves growth factors, adhesion molecules, and cellular receptors, and it is crucial for tumour cell survival, proliferation, and invasion [
151]. CD13, a zinc-dependent membrane-bound ectopeptidase, plays a critical role in angiogenesis [
152]; it is usually overexpressed in lung, breast, and prostate cancer.
Another research work was published in 2020 by Adrienn and colleagues in which the
68Ga-NODAGA-c(NGR) [
68Ga-c[Lys(1,4,7-Triazacyclononane,1-glutaric acid-4,7-acetic acid)-Asn-Gly-Arg-Glu]-NH
2] peptide was used to evaluate the efficacy of in vivo molecular imaging. The aim of that research was to determine the anti-tumour effects of bestatin and actinonin treatment in subcutaneously transplanted HT1080 and B16-F10 with the use of tumour-bearing animal models. Five days after injecting bestatin and actinonin, the PET scans showed that bestatin- and actinonin-treated B16-F10 tumours exhibited significantly low radiopharmaceutical uptake and accumulation. This means that the CD13 inhibitor may be suitable for suppressing the neoangiogenic process [
154].
Due to its high recurrence and mortality, ovarian cancer is a serious threat to women’s health. It is one of the most common gynaecological malignancies. Most patients with ovarian cancer show advanced intraperitoneal metastasis of the disease at prognosis, owing to its clinically silent nature [
159], and the differential overexpression of CD13 has been well-documented [
160]. With regard to the expression of APN/CD13, Yi Yang and colleagues reported on the use of the
68Ga-labelled dimeric cNGR peptide DOTA-c(NGR)
2 [DOTA, 1,4,7,10-tetraazacyclododecane-N,N′,N″,N‴-tetraacetic acid] for the micro-PET imaging of ovarian cancer xenografts. A higher uptake of
68Ga was found in ES2 cells when compared with SKOV3 cells, which makes their compound a candidate for the assessment of CD13 expression in ovarian cancer [
161]. In addition, many therapeutics have been delivered to active tumour neovascular tissue by peptides bearing the NGR motif as a homing agent. However, the affinity, specificity, and pharmacokinetics of radiolabelled NGR peptides from different molecular scaffolds and chelators vary greatly. Peptide dimerization targeting NGRs showed promise for the enhancement of pharmacokinetic properties and the tumour-to-nontarget ratio.
6. Radiolabelled Peptides Translated into Clinical Trials
Metastatic, castration-resistant prostate cancer is one of the most challenging curable diseases. Since castration-resistant prostate cancer does not respond to hormone therapy, there is an urgent need to develop a therapeutic agent that targets this type of cancer [162]. Recently, specific proteins, such as SWI/SNF, which are overexpressed in castration-resistant prostate cancer have been discovered [163]. Prostate-specific membrane antigen (PSMA) is also one of the proteins that is highly expressed in metastatic, castration-resistant prostate cancer [164]. In the clinical trial for the curing of this disease, 177Lu-PSMA-617 was investigated as a radioligand therapy that could deliver beta-particle radiation to PSMA-expressing cells and the surrounding microenvironment (NCT03511664) [164]. Oliver et al. evaluated the theragnostic efficacy of 177Lu-PSMA-617 through an international, open-label, phase 3 trial involving patients previously treated for metastatic, castration-resistant prostate cancer. The patients had undergone treatment of not only one androgen receptor pathway inhibitor, but also of one or two taxane regimens. Furthermore, PET-CT-scanned patients who used PSMA-positive gallium-68 (68Ga)-labelled PSMA-11 also participated. In the clinical trials, one group of patients additionally received 177Lu-PSMA-617 treatment (7.4 GBq every six weeks for four to six cycles) as they were provided with protocol-permitted standard care that excluded radium-223 (223Ra). The other group of patients only received standard care. The endpoints were determined based on bioimaging of the patients or the objective responses. The side effects that occurred during the clinical trial were limited to those that occurred within 30 days of the last administration of 177Lu-PSMA-617 and those that occurred before the subsequent anticancer treatment. According to the imaging-based results for progression-free survival and overall survival, 177Lu-PSMA-617 plus standard care led to significant improvement as compared with standard care alone (in the case of imaging-based progression-free survival, median was 8.7 vs. 3.4 months; hazard ratio for progression or death, 0.40; 99.2% confidence interval, 0.29 to 0.57; p < 0.001; in the case of survival, median was 15.3 vs. 11.3 months; hazard ratio for death, 0.62; 95% CI, 0.52 to 0.74; p < 0.001). However, the incidence of adverse events of grade 3 and above was higher with the administration of 177Lu-PSMA-617 (52.7%) than without (38.0%). Fortunately, the adverse events that occurred did not cause negative effects such as a reduction in the quality of life of the patients. Therefore, standard care plus radioligand therapy with 177Lu -PSMA-617 as a combination therapy significantly prolonged the imaging-based progression-free survival and overall survival of patients with PSMA-positive, metastatic, castration-resistant prostate cancer.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15030971