1. Circadian Rhythm Disruption
The most common disruptors of circadian rhythm in humans are lack of sleep, shift work, inadequate food timing, increased nocturnal activity, and use of electronic devices before bedtime [
36,
76]. Daylight saving time has also been shown to contribute to circadian rhythm disruption and, consequently, to MetS and CVD [
77,
78]. Improper timing of environmental cues, due to increased night-time activity and disrupted feeding patterns, shifts the phase of circadian rhythms by many hours in peripheral clocks, such as the liver, adipose tissue, and muscles, without significant effects on the suprachiasmatic nucleus (SCN) [
8]. This internal desynchrony in the organism, with central and peripheral clocks out of phase, has been associated with metabolic disorders [
8].
2. Treatment of MetS in Alignment with Circadian Rhythm
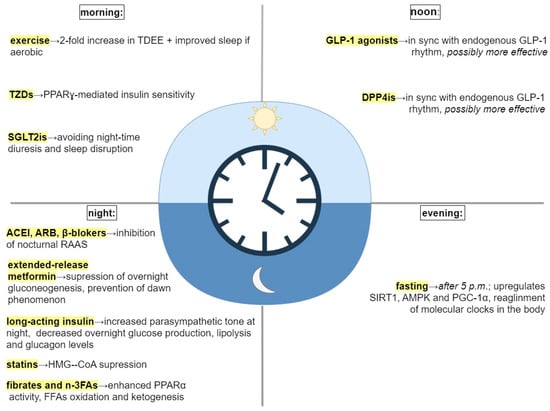
Restoration of disrupted circadian rhythm could improve routine treatment in patients with MetS. In accordance with the most recent findings of studies on chronotherapy, the proper timing of routine activities, such as feeding, exercise, and medication, is just as important as lifestyle and pharmacological interventions themselves [
9,
36,
41,
61,
66,
81,
83,
87,
88,
89,
90,
91] (
Figure 1).
Figure 1. Treatment interventions and their timing. Abbreviations: TDEE, total daily energy expenditure; TZDs, thiazolidinediones; PPARγ, proliferator−activated receptor γ; SGLT2is, sodium−glucose transport protein 2 inhibitors; GLP−1, glucagon−like peptid−1; DPP4is, dipeptidyl peptidase−4; SIRT1, sirtuin1; AMPK, adenosine monophosphate (AMP) activated protein kinase; PGC−1α, peroxisome proliferator-activated receptor−gamma coactivator 1α; ACEI, angiotensin−converting enzyme inhibitors; ARB, angiotensin receptor blocker; RAAS, renin−angiotensin−aldosterone system; HMG−CoA, β−hydroxy−3-methylglutaryl−CoA; n−3FAs, omega−3 fatty acids; PPARα, proliferator-activated receptor α; FFAs, free fatty acids.
2.1. Feeding According to the Biological Clock as a Circadian Rhythm Realignment Strategy
Time-restricted feeding (TRF) is a dietary strategy that consolidates all calorie intake into a daily window of 6 to 10 h [
61,
63,
100,
101]. Many studies demonstrate that TRF is a strong stimulus, capable of resetting both central and peripheral clocks [
36,
61,
63,
100,
101]. Time restriction of food availability promotes complex changes in the phase and amplitude of clock-controlled gene expression. Resynchronization of the rhythms happens during fasting [
9,
36]. Fasting promotes activation of sirtuin 1 (SIRT1), adenosine monophosphate (AMP) activated protein kinase (AMPK), and peroxisome proliferator-activated receptor-gamma coactivator 1α (PGC-1α) signaling pathways [
9]. This results in a phase advance and beginning of the new circadian cycle at the same time throughout the body [
9]. Increased AMPK activity promotes free fatty acids (FFA) oxidation and inhibits acetyl-CoA carboxylase (ACC), one of the enzymes involved in fat storage [
9]. SIRT1 improves insulin secretion and sensitivity, and suppresses inflammatory cytokine expression, which is an important component of MetS [
38]. PGC-1α enhances hepatic fatty acid oxidation, which has a positive effect on triglycerides (TG) levels [
43].
Parr et al. compared the effects of TRF (feeding window of 7 h/day, first meal consumed at 10 am, last meal of the day at 5 pm) vs. extended feeding (EXF; feeding window of 14 h/day, first meal at 07 am, last meal at 9 pm) on glucose and insulin levels, in overweight men [
92]. TRF improved nocturnal and postprandial blood glucose control [
92]. The 24-h total area under the curve (AUC total) for venous glucose appeared (
p = 0.09) to be lower for TRF compared with EXF (5.5 9.0 mmol/L/h), which was primarily caused by nocturnal (sleep) glucose AUC being lower in the TRF condition (−4.2 ± 5.8 mmol/L/h,
p = 0.04) [
92]. AUC total for venous insulin levels was generally lower in the TRF condition compared to EXF, although not substantially (
p = 0.11; 114 197 mIU/mL/h) [
92].
Considering the benefits of TRF, a question that imposes itself is: does the exact timing of the eating window matter? An early TRF, where the food intake occurs between 8 am and 6 pm, facilitates weight loss and appetite reduction in people with increased body mass index (BMI), and also has beneficial effects on insulin sensitivity, postprandial glycemia, lipid levels, and blood pressure (BP) [
62,
64,
87,
102]. On the other hand, restricting food intake to the late afternoon or evening (first meal after 12 pm) does not affect or even worsens these parameters [
62,
64,
87,
102]. In addition, late TRF results in inconsistent weight loss, ranging from a slight to no discernible change in weight and whole-body fat mass [
62,
64,
87,
102]. Considering all of these facts, TRF with food consumption limited to the early active phase (short feeding window of 7 h and first meal in the morning) could be an extremely beneficial treatment approach in patients with MetS [
102].
Furthermore, Jakubowitz et al., in their study, compared two isocaloric weight-loss groups and found that the group that received a larger breakfast and a smaller dinner showed higher improvement in metabolic indicators (body weight and BMI, waist circumference [WC], serum glucose, insulin, ghrelin, and lipids), compared with the group who received a smaller breakfast and a larger dinner [
103]. The larger breakfast group showed a 2.5-fold greater weight loss and greater reduction in WC [
103]. Fasting glucose, insulin and ghrelin also decreased to a greater extent in the larger breakfast group [
103]. After 12 weeks, mean serum TG concentrations decreased by 33.6% in the larger breakfast group, but increased by 14.6% in the larger dinner group [
103]. High-density lipoprotein (HDL) cholesterol slightly but significantly increased only in the group that received a larger breakfast [
103].
A prospective study conducted by Timlin et al. demonstrated that the frequency of breakfast among adolescents was inversely associated with BMI in a dose-response manner [
104]. Energy, carbohydrate, and fiber consumption were greater among breakfast eaters, but saturated fat consumption was lower [
104]. Those who regularly ate breakfast appeared to be substantially more physically active than those who skipped it [
104].
Wilkinson et al. assessed whether TRF can act synergistically with pharmacotherapy in a small cohort of patients with MetS who had an unrestricted eating pattern pre-trial [
105]. In this study, TRF (eating window 10 h/day for 12 weeks) showed an additive effect to pharmacotherapy (statins and/or anti-hypertensive treatment), by reducing WC, as well as whole-body and visceral fat, lowering BP, and decreasing glycosylated hemoglobin and serum lipids [
105]. After 12 weeks of TRF, the mean body weight and BMI reduction were both 3% [
105]. The decrease in body weight accompanied desirable reductions in body fat (3%) [
105] The mean WC reduction was 4% [
105]. The mean systolic and diastolic BP reduction were 4% and 8%, respectively [
105]. After 12 weeks, glycosylated hemoglobin dropped 1–3.7% from baseline [
105].
These results indicate that TRF should be added to the standard medical practice to treat MetS.
Intermittent fasting (IF) is an eating pattern which consists of changing regularly between periods of eating and fasting [
9,
106]. IF encompasses different fasting regimens, among which the most popular are alternate day fasting (ADF), which involves alternating 24 h of minimal food intake with 24 h of unrestricted intake, and the 5:2 diet, which includes regular food intake 5 days a week and 2 days fasting [
106]. Fasting activates multiple nutrient-responsive pathways, including the insulin/insulin-like growth factor (IGF-1) pathway and AMPK [
107]. It upregulates SIRT1 expression [
107]. Hepatic lipid droplets are targeted during intermittent fasting, which ultimately leads to TG hydrolysis via lysosomal acid lipase [
22], mediated by proliferator-activated receptor α (PPARα) [
108].
ADF has been shown to be effective in improving metabolic indicators in non-obese subjects [
109]. Following a 12-week ADF, subjects demonstrated a loss of 6.5 kg ± 1.0% in body weight and a reduction of 3.6 ±0.7 kg in fat mass, compared to controls [
109]. TG concentrations decreased by 20 ± 8%, and low-density lipoprotein (LDL) particle size increased in the ADF group relative to controls [
109]. C-reactive protein (CRP) decreased (13 ± 17%,
p < 0.05) in the ADF group relative to controls at week 12 [
109]. Plasma adiponectin increased (6 ± 10%,
p < 0.01), while leptin decreased (40 ± 7%,
p < 0.05), in the ADF group versus controls by the end of the study [
109].
ADF has a positive effect on obese subjects as well [
110]. After 10 weeks of ADF in obese individuals, body weight decreased by 5.6 ± 1.0 kg, percentage of body fat decreased by 3 ± 2%, total cholesterol, LDL, and TG concentrations decreased by 21 ± 4%, 25 ± 10%, and 32 ± 6%, respectively [
110]. HDL cholesterol remained unchanged. Systolic BP decreased from 124 ± 5 to 116 ± 3 mm Hg [
110].
Tripolt et al. examined glucose metabolism and metabolomics profiles after 12 h and 36 h fasting in non-obese and obese participants and people with type 2 diabetes mellitus (T2DM) [
111]. Fasting glucose was not significantly changed from baseline, but fasting insulin was significantly lower in both men and women (
p < 0.001) [
111]. Fasting β-hydroxybutyrate (BHB) and FFA concentrations were higher by the end of the study in both male and female subjects [
111]. HDL was elevated from baseline in the women only (
p < 0.001), and TGs were significantly reduced from baseline in the men only (
p < 0.05) [
111].
Kang et al. demonstrated that an IF 5:2 regimen produced superior weight loss (7.9 ± 5.0 kg), compared to daily caloric restriction (4.7 ± 3.4 kg), during a 12-week study on Chinese overweight and obese patients [
112].
Despite the fact that it has been shown as a beneficial treatment intervention for weight loss and the improvement of metabolic indicators, it is questionable whether IF could be applicable on a day-to-day basis, especially in elderly populations and in patients with T2DM. The common adverse effects of IF include dizziness, headache, nausea, irritability, hypoglycemia, and temporary sleep disturbances [
106,
112,
113]. Nevertheless, in studies with IF, adverse effects were less common and less severe over time, suggesting that the body takes some time to adapt [
106,
112,
113].
2.2. Exercise around the Clock
Current Standards of Medical Care in Diabetes by the American Heart Association recognize that overweight and obese people with an increased risk for developing T2DM should enroll into programs for lifestyle behavior change [
99]. They should introduce moderate-intensity aerobic activity, such as a brisk walk 150 min/week, and eat less fat and fewer calories with a goal to lose 7% of their initial body weight (grade A evidence) [
99]. Exercise timing has a bimodal on the circadian clock [
42]. Firstly, it acts as a time cue, crucial in the realignment of disrupted central and peripheral circadian clocks [
42].
Many proxies of circadian rhythm have been reported to be altered by exercise, including hormone secretion (e.g., melatonin, cortisol, and thyroid-stimulating hormone [TSH]) and physiological parameters (e.g., body temperature, BP) [
114,
115]. For instance, melatonin phase delays are associated with exercise in the evening or overnight [
115].
Secondly, exercise’s metabolic outputs might depend on its daily timing [
42].
Sato et al. examined how the timing of exercise impacts local tissue and systemic metabolism in mice [
35]. Exercise or control sham exercise was performed for 1 h on a treadmill at either early light/rest phase (ZT3) or early dark/active phase (ZT15), followed by the detection of metabolites such as ketones, amino acids (AAs), lipids in white adipose tissue (WAT), muscle, and serum [
35]. Exercise at ZT15, i.e., in the early active phase, increased the levels of BHB and urea, indicating a greater dependence on FFA oxidation and enhanced buffering against metabolic stress [
35]. Acyl-carnitine levels increased more upon ZT15 exercise in both muscle and serum, which suggests that exercise at ZT15 activates FFA oxidation in muscle and increases the demand for energy from non-glycolytic sources [
35]. These findings are confirmed by gene expression profiling, which found that muscle genes involved in FFA oxidation, including peroxisome proliferator-activated receptor δ (Pparδ), were specifically upregulated following exercise at ZT15 [
35]. Exercise at ZT15 also boosted muscle AMP levels and activated AMPK [
35]. Activated AMPK phosphorylates and destabilizes circadian transcriptional repressors CRY1/2 [
35]. This allows the de-repression of
Bmal1:Clock targets, which results in the reprogramming of the circadian and gluconeogenic genes [
35]. Thus, exercise may reset misaligned muscle clocks if timed appropriately, i.e., in the early-active phase [
35].
Asher et al. studied the variation in exercise capacity of wild-type mice between two distinct time points during their active phase, namely 2 h and 10 h within the dark phase [
116]. Exercise had a different impact on gene expression in the Early group compared to the Late group [
116]. Insulin-signaling pathways and glucose metabolism were enriched specifically in the Early group. Moreover, PPARα was upregulated only in the Early group [
116]. Following exercise, the Early group displayed a more pronounced decline in lipids and amino acids, compared to the Late group [
116].
Asher at al. also detected higher oxygen consumption in humans during exercise in the early phase (8 am) vs. late phase (6 pm) [
116]. Evening exercise is associated with greater exercise capacity and endurance, which is related to a greater reliance on carbohydrates and higher body temperature in the evening [
116]. Carbohydrates require less oxygen per amount of ATP produced, which may contribute to the lower oxygen consumption and lower glycemia upon evening exercise [
116]. On the other hand, muscle cells seem to be more effective at FFA oxidation in the morning, potentially leading to greater fat loss [
116]. Therefore, morning exercise could be an effective strategy for people with obesity and/or T2DM [
116].
In a study by Creasy at al., during A 15-week exercise protocol, morning exercise (6–10 am) resulted in a >2-fold increase in total body energy expenditure compared with evening exercise (5–7 pm) [
93]
According to research by Willis et al., obese young adults lost significantly more weight over the course of 10 months of high-intensity, supervised aerobic exercise (2000–3000 kcal/week) in the morning (−7.2 ± 1.2%;
p < 0.001) than they did in the evening (−2.1 ± 1.0%;
p < 0.001) [
65].
In a study performed by Tokuyama et al., 24 h FFA oxidation was the highest in participants who exercised in the morning before breakfast, compared with those who exercised in the afternoon, evening, and sedentary controls [
117]. Transient carbohydrate deficits, i.e., glycogen depletion observed after morning exercise, may have contributed to increased 24 h fat oxidation [
117].
Several studies demonstrate how aerobic exercise supports circadian alignment to optimize health outcomes [
94,
95,
96]. Van Someren and colleagues found that 3 months of consistent aerobic exercise, performed three times a week, counteracted age-related disruption of the circadian clock and improved sleep quality in otherwise healthy older men (73 ± 2 years) [
94]. A significant correlation was found between maximal aerobic fitness and reduced circadian variability after the intervention, an important finding given the association between aerobic capacity and all-cause mortality [
95,
96].
2.3. Circadian Medication
The availability of binding sites for medications used in the treatment of MetS shows circadian oscillations, and it is established that effectiveness, as well as toxicity, of these drugs varies depending on the specific time of the day [
118].
2.3.1. Antihypertensives
It is no wonder that chronotherapy has its roots in the treatment of hypertension, considering the fact that day–night variations in BP are among the best-known circadian rhythms of physiology [
83,
88,
89,
90,
119]. ACE inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), are proven to be more effective when administered in the evening, compared with morning-dosed controls [
83,
90,
119]. Their better efficacy at night can be explained by the inhibition of nocturnal renin-angiotensin-aldosterone system (RAAS) activation [
74].
Numerous studies support this theory [
83,
88,
89,
90,
119]. Kuroda et al. demonstrated that the long-acting ACEI trandolapril was a safer and more effective after bedtime administration, compared with morning administration [
88]. Hermida et al. demonstrated that bedtime spirapril administration was more efficient in BP control during both nocturnal sleep and daytime activity, compared with morning administration [
119]. In another study by Hermida et al., the reduction in the 48 h mean values for systolic and diastolic BP was greatest when a valsartan/amlodipine combination was ingested at bedtime [
89]. Moreover, in a study on non-dipper patients with essential hypertension (grade 1–2), valsartan administration at bedtime, as opposed to upon awakening, showed improved efficacy in BP control during the nocturnal resting hours [
83]. In this study, 75% of the patients in this group reverted to dippers, there was a significant increase in the percentage of patients with controlled BP over 24 h, and there was a reduction in urinary albumin excretion [
83].
There are no papers on the timing of other groups of antihypertensives, to the best of our knowledge.
2.3.2. Antihyperglycemic Medications
Metformin
Metformin is a widely-used treatment option for “prediabetes”, i.e., IR, in MetS, and its mechanism of action has been linked to the activation of AMPK, a positive-loop regulator of the circadian rhythm [
41]. AMPK promotes the degradation of PER2 through the activation of casein kinase I isoform epsilon (CK1ε), which leads to the phase advance of circadian clocks in the peripheral tissues [
41]. Metformin could also have beneficial effects against oxidative injury, given that it activates PGC-1α via AMPK induction, to restore the mitochondrial network and to counteract reactive oxygen species (ROS) generation [
44].
The effects of metformin are tightly linked to circadian rhythm, which emphasizes the importance of proper timing of metformin administration [
41]. Extended-release metformin should be taken once a day at night, with dinner. Bedtime administration is proven to be the most effective in blood glucose regulation, given that, by targeting clock genes, metformin suppresses overnight gluconeogenesis and prevents morning hyperglycemia [
41].
Imeglimin
Imeglimin (IMEG), the first of the group of oral tetrahydrotriazine compounds, has also been found to activate AMPK, but to a lesser extent, compared to metformin [
120]. A recent study by Hozumi and colleagues found that IMEG reduced the ATP/ADP ratio in primary cultured hepatocytes, which was the most probable trigger for AMPK activation [
120]. The measured AMPK rates, however, were lower than those induced by metformin [
120]. Although IMEG exerts similar effects to those of metformin on AMPK activity, it is yet to be determined whether it is potent enough to phase-shift the circadian rhythms [
120]. IMEG is currently administered twice daily, in the morning and evening [
121], but further studies are needed to determine whether IMEG’s effects depend on the time of day it is administered.
PPARγ Agonists
Thiazolidinediones (TZDs), as full agonists of PPARγ, have been shown to improve insulin sensitivity [
97]. They affect catabolism of TGs to a lesser extent [
97]. They could also have beneficial effects on the CV system, given that PPARγ plays essential role in maintaining the circadian rhythms of BP and heart rate [
97].
A recent study in mice showed that pioglitazone can resynchronize clock genes and inflammation-related genes in the mouse liver that have been disrupted by reverse feeding, i.e., feeding during the rest phase [
91]. This resynchronization resulted in decreased hyperglycemia, hypercholesterolemia, and transaminase activity, as well as decreased interleukin 6 ( IL-6) in liver tissue [
91]. It is yet to be determined whether the same results can be achieved in humans.
PPARγ expression in adipose tissue and skeletal muscle peaks at the beginning of the active phase [
20]. This means that morning administration of TZDs could be more efficient in insulin resistance (IR) treatment. Further research is needed to provide more evidence in support of this hypothesis.
GLP-1 RAs
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) offer pharmaceutical levels of GLP-1, which lower blood sugar levels and body weight, by enhancing glucose-dependent insulin secretion, decreasing glucagon secretion, prolonging gastric emptying, and inducing satiety [
69].
Although, to the best of our knowledge, there are still no studies related to this subject, it is possible that GLP-1 RAs, such as liraglutide and lixisenatide, could have a stronger beneficial effect if administered in sync with endogenous GLP-1 rhythms.
DPP-4is
Dipeptidyl peptidase (
DPP-
4) inhibitors (DPP-4is) are antihyperglycemic drugs which prevent the inactivation of GLP-1, thereby increasing its levels and potentiating its action [
72].
Although, to the best of our knowledge, there are still no studies related to this subject, DPP-4is could have a stronger beneficial effect if administered in sync with endogenous GLP-1 rhythms [
69].
SGLT2 Inhibitors
Sodium-glucose cotransporter-2 inhibitors (SGLT2is) inhibit renal glucose reabsorption by blocking the SGLT2 cotransporters in the proximal tubules and causing glucosuria [
122]. The accompanying sodium excretion explains their additional effect on lowering BP [
122]. Their implication with regard to the circadian pressure has been shown, and emerging data suggest that SGLT2is not only decrease BP, but also improve its disrupted circadian rhythm [
98]. Nevertheless, there is no significant difference in the effectiveness of SGLT2is between morning and evening administration [
123]. Evening administration might be less favorable because these medications increase urinary volume and disrupt night-time sleep with frequent bathroom visits, which might negatively affect circadian balance [
98].
Insulin
Porcellati and colleagues observed significant differences in the pharmacokinetics and pharmacodynamics of basal insulin glargine after morning vs. evening administration [
124]. With morning or evening glargine dosing, total insulin activity on glucose metabolism was similar [
124]. Evening glargine administration, however, consistently reduced nocturnal endogenous glucose production, lipolysis, and glucagon concentration [
124]. Thus, compared to morning glargine dosing, targeting fasting euglycemia with evening glargine dosing seems more convenient [
124].
A study by Takeshita et al. compared the effects of two different insulin regimens—basal (insulin glargine) versus bolus insulin (glulisine)—on metabolic and cardiovascular autonomic function in Japanese participants with T2DM [
125]. Insulin glargine, but not insulin glulisine, increased parasympathetic tone during night-time and decreased sympathetic nerve activity at dawn [
125]. These findings shed light on the previously unrecognized role of night-time basal insulin supplementation on sympatho-vagal circadian rhythm in T2DM [
125].
2.3.3. Hypolipidemic Agents
Statins
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are considered a standard therapy for many types of dyslipidemia. Circadian-regulated statin administration was established decades ago [
81]. They are generally administered in the evening, because HMG-CoA reductase-regulated cholesterol biosynthesis peaks during the night [
81]. Awad and colleagues found that short-acting statins taken in the evening were significantly more effective in lowering LDL-C and total cholesterol, consequently reducing CVD risk, than those taken in the morning [
81]. Furthermore, in a randomized control trial by Wallace et al., simvastatin was found to be significantly more effective in lowering LDL-C and total cholesterol when taken at night [
126].
PPARα Agonists
PPARα agonists (fibrates) and omega-3 fatty acids are powerful TG-lowering agents. They affect TG catabolism by promoting β-oxidation and raising the levels of HDL [
127]. They are also powerful stimulators of ketogenesis [
128]. One of their downsides is the contribution to gallstone formation [
129]. They inhibit bile acid synthesis, via PPARα-mediated downregulation of cholesterol 7α-hydroxylase and sterol 27-hydroxylase [
129]. It is known that these rate-limiting enzymes exhibit diurnal rhythmicity, with the highest activity at noon [
130]. The current recommendation is to take fibrates with a meal, once a day [
131]. If fibrates were to be taken at night, they would enhance physiological PPARα night-time activity and physiological TG catabolism [
86,
132]. On the contrary, when taken during the day, they would activate PPARα at the wrong time and place, which would result in the suppression of bile acid synthesis and, thus, a higher probability of gallstone formation [
86,
132].
PPARβ/δ Agonists
In addition to the insulin-sensitizing and antihyperglycemic effects of TZDs, PPARβ/δ agonists, such as elafibranor and telmisartan, achieve TG-lowering and HDL-raising effects [
91]. They also have potential for treating NAFLD, which is closely related to MetS [
91]. Telmisartan is also ARB and is widely used in the treatment of hypertension [
90]. Its antihypertensive effect is significantly better with bedtime dosing [
90]. Telmisartan could potentially be an optimal medication for patients with MetS, considering its beneficial effects on insulin sensitivity, lipid levels, and hypertension.
To the best of our knowledge, there is no data on the timing of administration of other hypolipidemic agents, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and ezetimibe.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11041171