Marine sponges are multicellular and primitive animals that potentially represent a wealthy source of novel drugs. The genus Acanthella (family Axinellidae) is renowned to produce various metabolites with various structural characteristics and bioactivities, including nitrogen-containing terpenoids, alkaloids, and sterols. Different metabolites were separated and characterized from different species of this genus using various spectroscopic and chromatographic techniques. The isolated metabolites are categorized according to their chemical classes into sesquiterpenes, diterpenes, alkaloids, steroid compounds, and others. Additionally, their reported biosynthetic and synthetic studies are also highlighted whenever applicable.
- Acanthella
- Axinellidae
- nitrogen-containing terpenoids
- Life below water
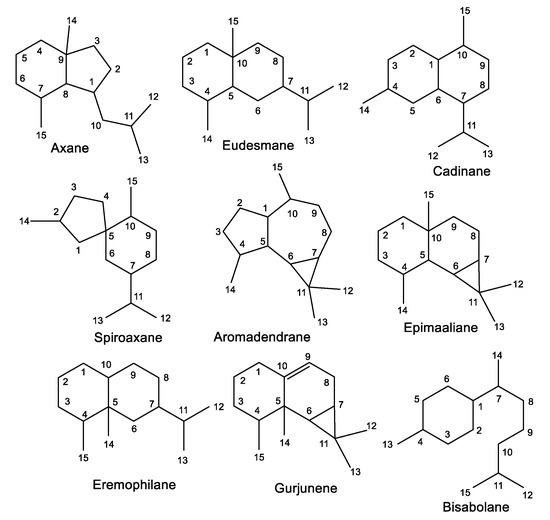
1. Sesquiterpenes
| Compound Name | Mol. Wt. | Mol. Formula | Species | Sampling Locations | Ref. |
|---|---|---|---|---|---|
| Aromadendrane-type sesquiterpenes | |||||
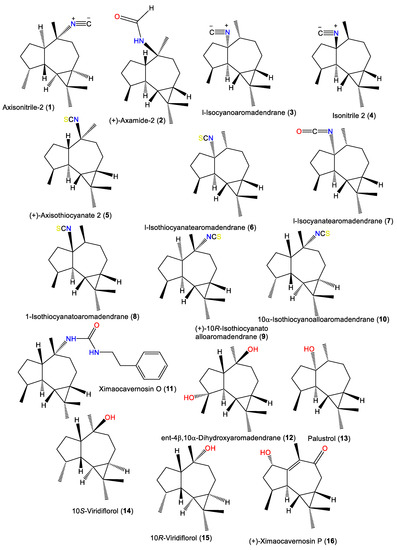
| Axisonitrile-2 (1) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [32] |
| (+)-Axamide 2 (2) | 249 | C16H27NO | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| l-Isocyanoaromadendrane (3) | 231 | C16H25N | A. acuta | Near Banyuls, France | [34,35] |
| - | - | A. pulcherrima | Weed Reef, Darwin, Australia | [35] | |
| - | - | A. acuta | Near Banyuls, France | [36] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [37] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| Isonitrile 2 (4) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [39] |
| (+)-Axisothiocyanate 2 (5) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| l-Isothiocyanatearomadendrane (6) | 263 | C16H25NS | A. acuta | Near Banyuls, France | [36] |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [37] | |
| 1-Isocyanatearomadendrane (7) | 247 | C16H25NO | A. acuta | Near Banyuls, France | [36] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| 1-Isothiocyanatoaromadendrane (8) | 263 | C16H25NS | A. acuta | Bay of Naples, southern Italy | [39] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| (+)-10R-Isothiocyanatoalloaromadendrane (9) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [42] | |
| - | - | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] | |
| 10α-Isothiocyanoalloaromadendrane (10) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [37] | |
| Ximaocavernosin O (11) | 368 | C24H36N2O | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
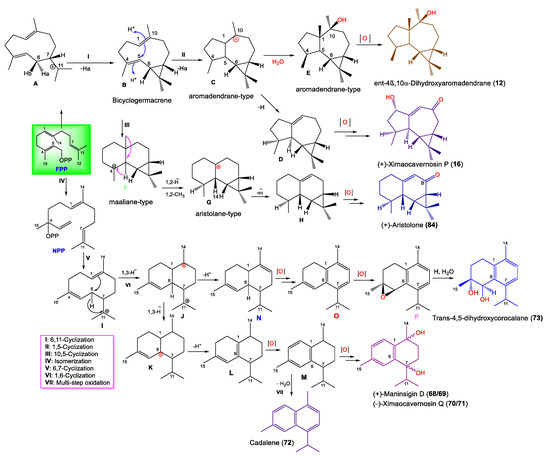
| ent-4β,10α-Dihydroxyaromadendrane (12) | 238 | C15H26O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| Palustrol (13) | 222 | C15H26O | A. acuta | Near Banyuls, France | [34] |
| - | - | A. acuta | Near Banyuls, France | [36] | |
| 10S-Viridiflorol (14) | 222 | C15H26O | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| 10R-Viridiflorol (15) | 222 | C15H26O | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| (+)-Ximaocavernosin P (16) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| Spiroaxane-type sesquiterpenes | |||||
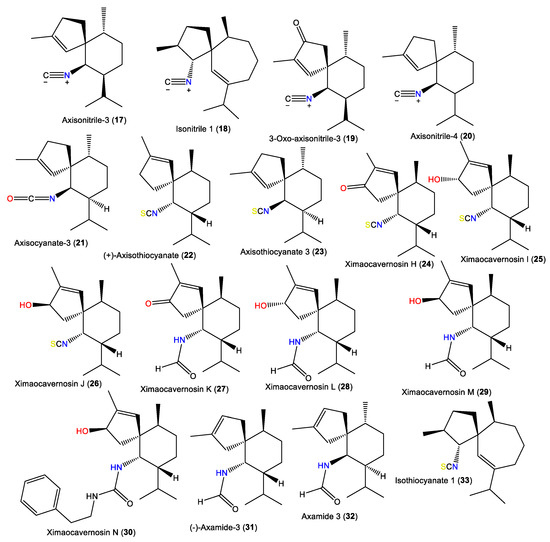
| Axisonitrile-3 (17) | 231 | C16H25N | A. acuta | Near Banyuls, France | [34] |
| - | - | A. cavernosa | Thailand | [18] | |
| - | - | A. klethra | Pelorus Island, Queensland, Australia | [19] | |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [44] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [31] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [37] | |
| Isonitrile 1 (18) | 245 | C17H27N | A. acuta | Bay of Naples, southern Italy | [39] |
| 3-Oxoaxisonitrile-3 (19) | 245 | C16H23NO | Acanthella sp. | Ximao Sea, Hainan, China | [41] |
| Axisonitrile-4 (20) | 231 | C16H25N | A. acuta | Sidi Elghdamssi island, Monastir region, Tunisia | [25] |
| Axisocyanate-3 (21) | 247 | C16H25NO | A. cavernosa | Tani’s Reef, Mooloolaba, Australia | [37] |
| (+)-Axisothiocyanate (22) | 263 | C16H25NS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Axisothiocyanate 3 (23) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [19] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [44] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [40] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [31] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| Ximaocavernosin H (24) | 277 | C16H22NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin I (25) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin J (26) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin K (27) | 263 | C16H25NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin L (28) | 265 | C16H27NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin M (29) | 265 | C16H27NO2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Ximaocavernosin N (30) | 368 | C24H36N2O | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (–)-Axamide 3 (31) | 249 | C16H27NO | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Axamide 3 (32) | 249 | C16H27NO | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| Isothiocyanate 1 (33) | 277 | C17H27NS | A. acuta | Bay of Naples, southern Italy | [39] |
| Eudesmane-type sesquiterpenes | |||||
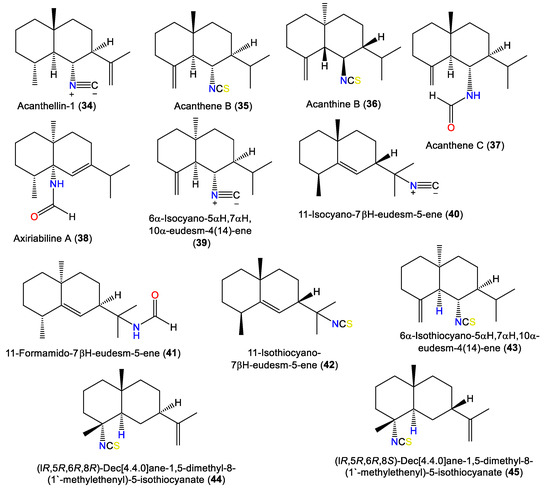
| Acanthellin-1 (34) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [30] |
| - | - | A. acuta | Near Banyuls, France | [34] | |
| - | - | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [47] | |
| - | - | A. acuta | Sidi Elghdamssi Island, Monastir region, Tunisia | [25] | |
| Acanthene B (35) | 263 | C16H25NS | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| Acanthine B (36) | 263 | C16H25NS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Acanthene C (37) | 249 | C16H27NO | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| Axiriabiline A (38) | 249 | C16H27NO | A. cavernosa | Xidao Island, Hainan, China | [48] |
| 6α-Isocyano-5αH,7αH,10α-eudesm-4(14)-ene) (39) | 231 | C16H25N | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [47] |
| - | - | A. acuta | Bay of Naples, southern Italy | [39] | |
| 11-Isocyano-7βH-eudesm-5-ene (40) | 231 | C16H25N | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| 11-Formamido-7βH-eudesm-5-ene (41) | 249 | C16H27NO | A. cavernosa | Xidao Island, Hainan, China | [48] |
| 11-Isothiocyano-7βH-eudesm-5-ene (42) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| - | - | A. klethra | Pelorus Island, Queensland, Australia | [19] | |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [44] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [31] | |
| 6α-Isothiocyano-5αH,7αH,10α-eudesm-4(14)-ene (43) | 263 | C16H25NS | A. acuta | Bay of Taranto near Porto Cesareo, Southern Italy | [47] |
| - | - | A. acuta | Bay of Naples, southern Italy | [39] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| (lR,5R,6R,8R)-Dec[4.4.0]ane-1,5-dimethyl-8-(1′-methylethenyl)-5-isothiocyanate (44) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [19] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [44] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| (lR,5R,6R,8S)-Dec[4.4.0]ane-1,5-dimethyl-8-(1′-methylethenyl)-5-isothiocyanate (45) | 263 | C16H25NS | A. klethra | Pelorus Island, Queensland, Australia | [19] |
| - | - | A. klethra | Vicinities of Phantom and Pelom Islands, Queensland, Australia | [44] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| Cadinene-type sesquiterpenes | |||||
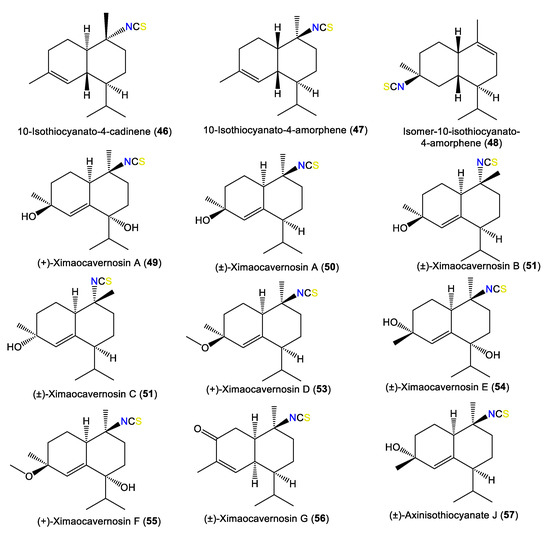
| 10-Isothiocyanato-4-cadinene (46) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| 10-Isothiocyanato-4-amorphene (47) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [49] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| Isomer-10-isothiocyanato-4-amorphene (48) | 263 | C16H25NS | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| (+)-Ximaocavernosin A (49) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| (±)-Ximaocavernosin A (50) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (±)-Ximaocavernosin B (51) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (±)-Ximaocavernosin C (52) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (+)-Ximaocavernosin D (53) | 293 | C17H27NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (±)-Ximaocavernosin E (54) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (+)-Ximaocavernosin F (55) | 309 | C17H27NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (±)-Ximaocavernosin G (56) | 277 | C16H23NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| (±)-Axinisothiocyanate J (57) | 279 | C16H25NOS | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
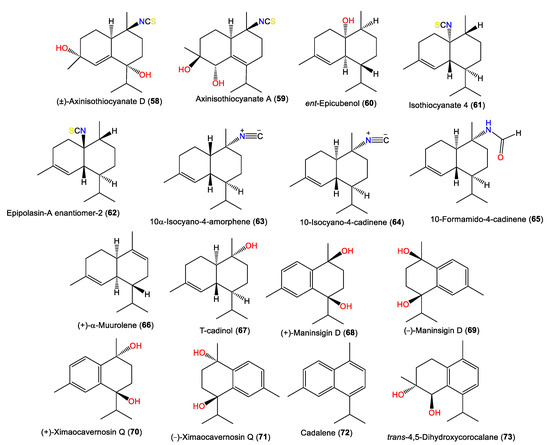
| (±)-Axinisothiocyanate D (58) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| Axinisothiocyanate A (59) | 295 | C16H25NO2S | A. cavernosa | Coast of Ximao Island, Hainan, China | [33] |
| ent-Epicubenol (60) | 222 | C15H26O | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| Isothiocyanate 4 (61) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| Epipolasin-A enantiomer-2 (62) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| 10α-Isocyano-4-amorphene (63) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [32] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [49] | |
| 10-Isocyano-4-cadinene (64) | 231 | C16H25N | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| - | - | A. cavernosa | Several locations off the Japanese coast | [49] | |
| - | - | A. cavernosa | Tani’s Reef or Coral Gardens dive sites | [38] | |
| 10-Formamido-4-cadinene (65) | 249 | C16H27NO | A. cavernosa | Several locations off the Japanese coast | [49] |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [48] | |
| (+)-α-Muurolene (66) | 204 | C15H24 | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| T-cadinol (67) | 222 | C15H26O | A. cavernosa | Several locations off the Japanese coast | [49] |
| (+)-Maninsigin D (68) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] | |
| (-)-Maninsigin D (69) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| (+)-Ximaocavernosin Q (70) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| (-)-Ximaocavernosin Q (71) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| Cadalene (72) | 198 | C15H18 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| trans-4,5-Dihydroxycorocalane (73) | 234 | C15H22O2 | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| Axane-type sesquiterpenes | |||||
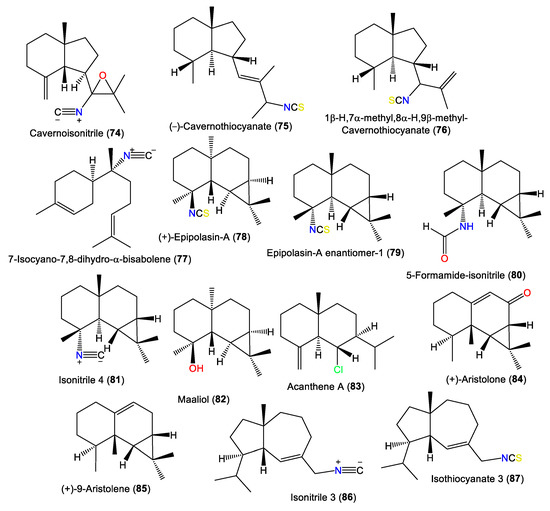
| Cavernoisonitrile (74) | 245 | C16H23NO | A. cavernosa | Hachijo-Jima Island, Japan | [32] |
| (-)-Cavernothiocyanate (75) | 263 | C16H25NS | A. cavernosa | Hachijo-Jima Island, Japan | [32] |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [40] | |
| 1β-H,7α-methyl,8α-H,9β-methyl-Cavernothiocyanate (76) | 263 | C16H25NS | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| Bisabolene-type sesquiterpenes | |||||
| 7-Isocyano-7,8-dihydro-α-bisabolene (77) | 231 | C16H25N | A. cavernosa | Hachijo-Jima Island, Japan | [32] |
| Epimaaliane-type sesquiterpenes | |||||
| (+)-Epipolasin-A (78) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [31] | |
| Epipolasin-A enantiomer-1 (79) | 263 | C16H25NS | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| 5-Formamide-isonitrile (80) | 249 | C16H27NO | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| Isonitrile 4 (81) | 231 | C16H25N | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| Maaliol (82) | 222 | C15H26O | A. pulcherrima | Weed Reef, Darwin, Australia | [35] |
| Acanthene A (83) | 240 | C15H25Cl | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] |
| Gurjunene | |||||
| (+)-Aristolone (84) | 218 | C15H22O | A. cavernosa | Coast of Ximao Island, Hainan, China | [43] |
| - | - | A. cavernosa | South China Sea | [50] | |
| (+)-9-Aristolene (85) | 204 | C15H24 | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| Isonitrile 3 (86) | 231 | C16H25N | A. acuta | Bay of Naples, southern Italy | [39] |
| Isothiocyanate 3 (87) | 263 | C16H25NS | A. acuta | Bay of Naples, southern Italy | [39] |
1.1. Aromadendrane-Type Sesquiterpenes
1.2. Spiroaxane-Type Sesquiterpenes
1.3. Eudesmane-Type Sesquiterpenes
1.4. Cadinene-Type Sesquiterpenes
1.5. Other Sesquiterpenes
2. Diterpenoids
| Compound Name | Mol. Wt. | Mol. Formula | Species | Sampling Locations | Ref. |
|---|---|---|---|---|---|
| Kalihinols | |||||
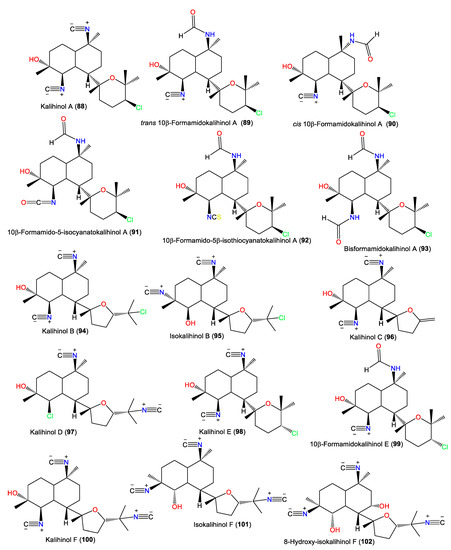
| Kalihinol A (88) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [52] |
| - | - | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] | |
| - | - | A. caruenosa | Fiji, South Pacific Ocean | [53] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [23] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [51] | |
| - | - | A. cavernosa | Yakushima Island, southwest of Tokyo | [55,56] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japa | [57] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| - | - | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [58] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [42] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| - | - | A. cavernosa | South China Sea | [50] | |
| trans 10β-Formamidokalihinol A (89) | 410 | C22H35ClN2O3 | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| - | - | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [58] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| cis 10β-Formamidokalihinol A (90) | 410 | C22H35ClN2O3 | A. cavernosa | Shallow water reef in Sanya Bay, Hainan Island, China | [58] |
| 10β-Formamido-5-isocyanatokalihinol A (91) | 426 | C22H35ClN2O4 | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [48] | |
| 10β-Formamido-5β-isothiocyanatokalihinol A (92) | 442 | C22H35ClN2O3S | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| - | - | A. cavernosa | Xidao Island, Hainan, China | [48] | |
| Bisformamidokalihinol A (93) | 428 | C22H37ClN2O4 | A. cavernosa | Xidao Island, Hainan, China | [48] |
| Kalihinol B (94) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [23] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| Isokalihinol B (95) | 392 | C22H33ClN2O2 | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [23] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] | |
| Kalihinol C (96) | 328 | C20H28N2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| Kalihinol D (97) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [42] | |
| Kalihinol E (98) | 392 | C22H35ClN2O3 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [40] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| - | - | A. cavernosa | South China Sea | [50] | |
| 10β-Formamidokalihinol E (99) | 410 | C22H33ClN2O2 | A. cavernosa | Hachijo-jima Island, Japan | [40] |
| Kalihinol F (100) | 383 | C23H33N3O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. caruenosa | Fiji, South Pacific Ocean | [53] | |
| - | - | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [60] | |
| - | - | Acanthella sp. | Queen Charlotte Island chain off the coast of British Columbia. | [46] | |
| - | - | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [23] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] | |
| - | - | Acanthella sp. | Coast of Cape Sada, Ehime Prefecture, Japan | [61] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| Isokalihinol F (101) | 383 | C23H33N3O2 | A. cavernosa | Fiji, South Pacific Ocean | [53] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [51] | |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| 8-Hydroxy-isokalihinol F (102) | 399 | C23H33N3O3 | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| 10-epi-Isokalihinol F (103) | 383 | C23H33N3O2 | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| trans 10-Formamido-kalihinol F (104) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [21] |
| cis 10-Formamido-kalihinol F (105) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [21] |
| trans 15-Formamido-kalihinol F (106) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [21] |
| cis 15-Formamido-kalihinol F (107) | 401 | C23H35N3O3 | A. cavernosa | Dibud, Philippines | [21] |
| Kalihinol G (108) | 415 | C23H33N3O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| 10-Isothiocyanatokalihinol G (109) | 447 | C23H33N3O2S2 | A. cavernosa | Xisha Islets, South China Sea | [16] |
| 5,10-bis-Isothiocyanatokalihinol G (110) | 479 | C23H33N3O2S3 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] |
| Kalihinol H (111) | 415 | C23H33N3O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| Isokalihinol H (112) | 415 | C23H33N3O2S | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| 10-epi-Isokalihinol H (113) | 415 | C23H33N3O2S | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| Kalihinol I (114) | 456 | C22H33ClN2O2S2 | A. cavernosa | Thailand | [18] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| 10-epi-Kalihinol I (115) | 456 | C22H33ClN2O2S2 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| - | - | A. cavernosa | South China Sea | [50] | |
| Kalihinol J (116) | 442 | C22H35ClN2O3S | A. cavernosa | Thailand | [18] |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
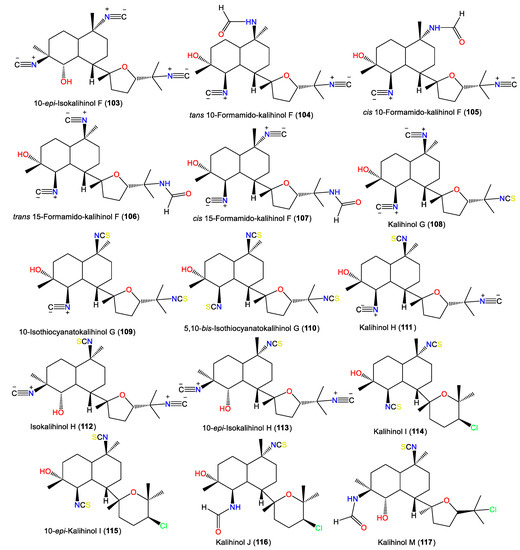
| Kalihinol M (117) | 442 | C22H35ClN2O3S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol N (118) | 442 | C22H35ClN2O3S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol O (119) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol P (120) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol Q (121) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol R (122) | 456 | C22H33ClN2O2S2 | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol S (123) | 410 | C22H35ClN2O3 | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol T (124) | 424 | C22H33ClN2O2S | A. cavernosa | Xisha Islets, South China Sea | [16] |
| Kalihinol X (125) | 424 | C22H33ClN2O2S | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [53] | |
| - | - | A. cavernosa | Thailand | [18] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| - | - | Acanthella sp. | Yalong Bay, Hainan, China | [42] | |
| 10-epi-Kalihinol X (126) | 424 | C22H33ClN2O2S | Acanthella sp. | Yalong Bay, Hainan, China | [42] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [16] | |
| - | - | A. cavernosa | South China Sea | [50] | |
| Kalihinol Y (127) | 365 | C21H32ClNO2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | Acanthella caruenosa | Fiji, South Pacific Ocean | [53] | |
| - | - | A. cavernosa | Thailand | [18] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| Kalihinone Ya (128) | 367 | C20H30ClNO3 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| Δ9-Kalihinol Y (129) | 365 | C21H32ClNO2 | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] |
| Kalihinol Z (130) | 392 | C22H33ClN2O2 | Acanthella sp. | Apra Harbor, Guam, western side of the USA | [22] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [53] | |
| - | - | A. cavernosa | Seychelles and Desnceufs Islands | [54] | |
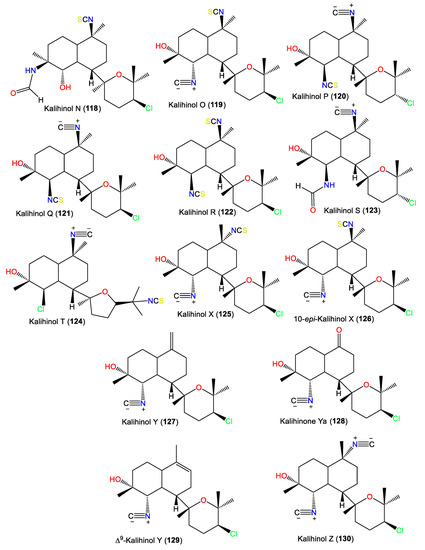
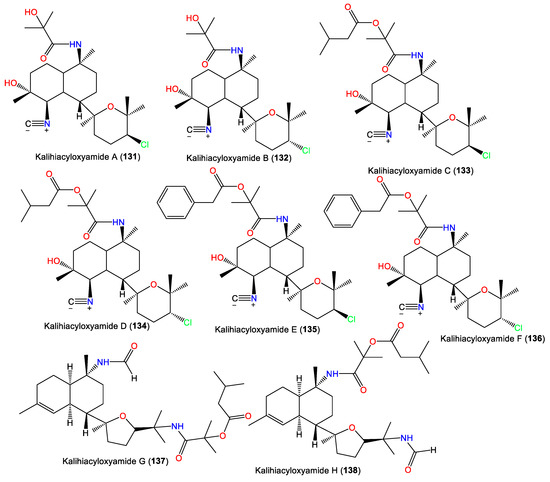
| Kalihiacyloxyamide A (131) | 468 | C25H41ClN2O4 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide B (132) | 468 | C25H41ClN2O4 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide C (133) | 552 | C30H49ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide D (134) | 552 | C30H49ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide E (135) | 586 | C33H47ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide F (136) | 586 | C33H47ClN2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide G (137) | 518 | C30H50N2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihiacyloxyamide H (138) | 518 | C30H50N2O5 | A. cavernosa | Xisha Island, South China Sea | [62] |
| Kalihinene | |||||
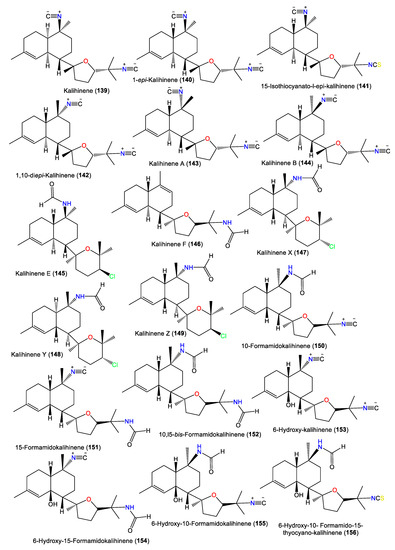
| Kalihinene (139) | 340 | C22H32N2O | A. klethra | Kuchinoerabu Island of the Satsunan Archipelago, Japan | [23] |
| - | - | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] | |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [51] | |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| - | - | A. cavernosa | Dibud, Philippines | [21] | |
| - | - | Acanthella sp. | Ximao Sea, Hainan, China | [41] | |
| 1-epi-Kalihinene (140) | 340 | C22H32N2O | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| 15-Isothiocyanato-l-epi-kalihinene (141) | 372 | C22H32N2OS | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| 1,10-diepi-Kalihinene (142) | 340 | C22H32N2O | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| Kalihinene A (143) | 340 | C22H32N2O | A. cavernosa | Seychelles and Desnceufs Islands | [54] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [63] | |
| Kalihinene B (144) | 340 | C22H32N2O | A. cavernosa | Seychelles and Desnceufs Islands | [54] |
| Kalihinene E (145) | 367 | C21H34ClNO2 | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Kalihinene F (146) | 331 | C21H33NO2 | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Kalihinene X (147) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55,56] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [63] | |
| Kalihinene Y (148) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55,56] |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [63] | |
| Kalihinene Z (149) | 367 | C21H34ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55,56] |
| 10-Formamidokalihinene (150) | 358 | C22H34N2O2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55,56] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [51] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [63] | |
| 15-Formamidokalihinene (151) | 358 | C22H34N2O2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55] |
| - | - | A. cavernosa | Fiji, South Pacific Ocean | [51] | |
| - | - | A. cavernosa | Xisha Islets, South China Sea | [63] | |
| 10,l5-bis-Formamidokalihinene (152) | 376 | C22H36N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [51] |
| 6-Hydroxy-kalihinene (153) | 356 | C22H32N2O2 | A. cavernosa | Fiji, South Pacific Ocean | [51] |
| - | - | Acanthella sp. | Coral reef of Ishigaki Island, Okinawa, Japan | [57] | |
| 6-Hydroxy-15-Formamidokalihinene (154) | 374 | C22H34N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [51] |
| 6-Hydroxy-10-Formamidokalihinene (155) | 374 | C22H34N2O3 | A. cavernosa | Fiji, South Pacific Ocean | [51] |
| 6-Hydroxy-10-Formamido-15-thyocyano-kalihinene (156) | 406 | C22H34N2O3S | A. cavernosa | Fiji, South Pacific Ocean | [51] |
| Kalihioxepanes | |||||
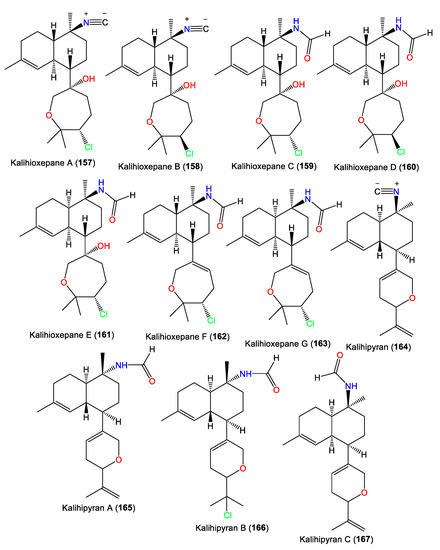
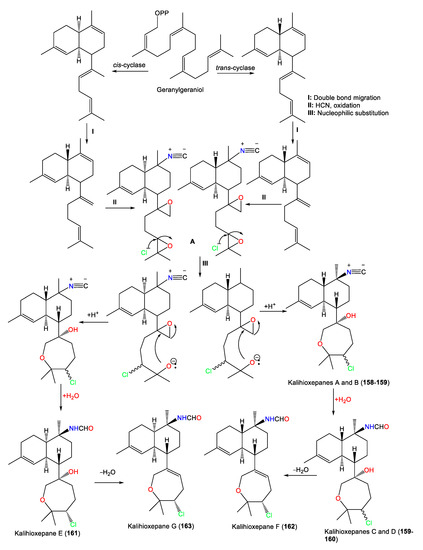
| Kalihioxepane A (157) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane B (158) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane C (159) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane D (160) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane E (161) | 383 | C21H34ClNO3 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane F (162) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [64] |
| Kalihioxepane G (163) | 365 | C21H32ClNO2 | A. cavernosa | Xisha Island, South China Sea | [64] |
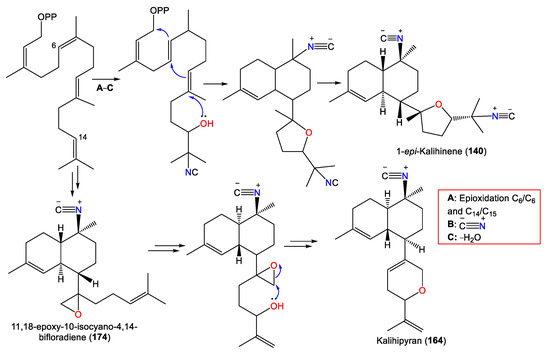
| Kalihipyran (164) | 311 | C21H29NO | A. cavernosa | Beau Vallon Beach, Mahé, Seychelles | [59] |
| - | - | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] | |
| Kalihipyrans | |||||
| Kalihipyran A (165) | 329 | C21H31NO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55] |
| Kalihipyran B (166) | 365 | C21H32ClNO2 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55] |
| Kalihipyran C (167) | 329 | C21H31NO2 | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Biflorane diterpenes | |||||
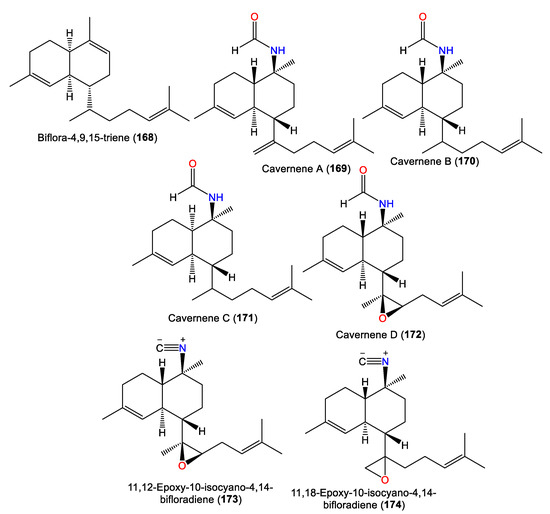
| Biflora-4,9,15-triene (168) | 272 | C20H32 | A. cavernosa | Yakushima Island, southwest of Tokyo | [55] |
| - | - | A. cavernosa | Hachijo-jima Island, Japan | [40] | |
| Cavernene A (169) | 315 | C21H33NO | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Cavernene B (170) | 317 | C21H35NO | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Cavernene C (171) | 317 | C21H35NO | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Cavernene D (172) | 331 | C21H33NO2 | A. cavernosa | Xisha Islets, South China Sea | [63] |
| Isocyanobifloradiene epoxide A (173) 11,12-epoxy-10-isocyano-4,14-bifloradiene (173) |
313 | C21H31NO | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
| Isocyanobifloradiene epoxide B (174) 11,18-epoxy-10-isocyano-4,14-bifloradiene (174) |
313 | C21H31NO | A. cavernosa | Heron Island, Great Barrier Reef, Australia | [45] |
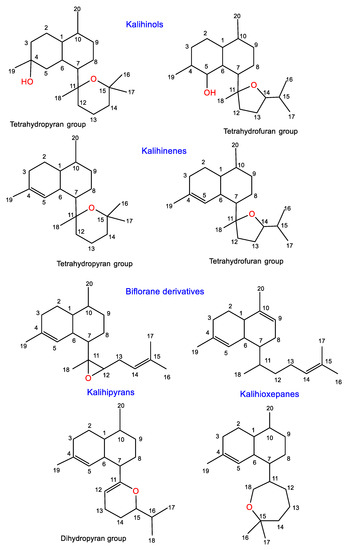
2.1. Kalihinols
2.2. Kalihinenes
2.3. Kalihipyrans and Kalihioxepanes
2.4. Biflorane Diterpenes
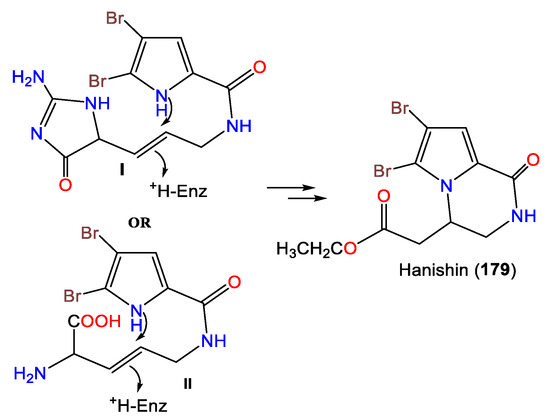
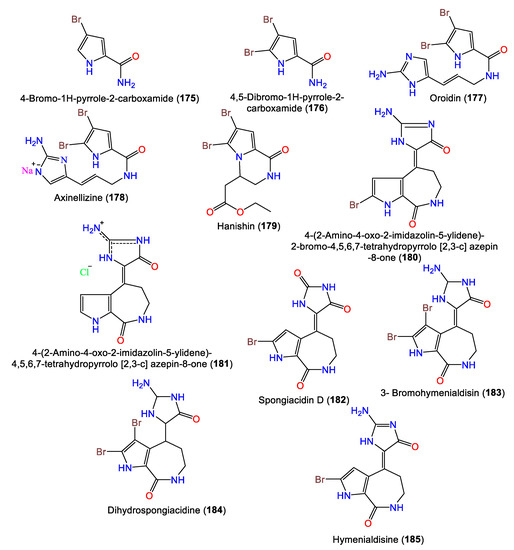
3. Alkaloids
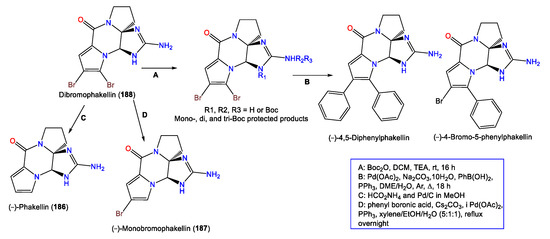
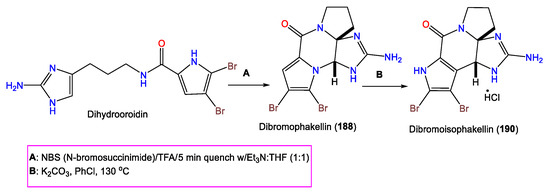
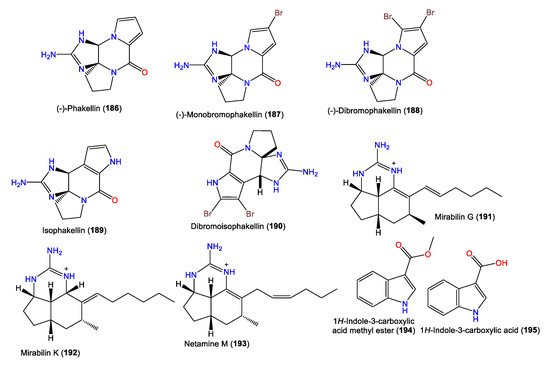
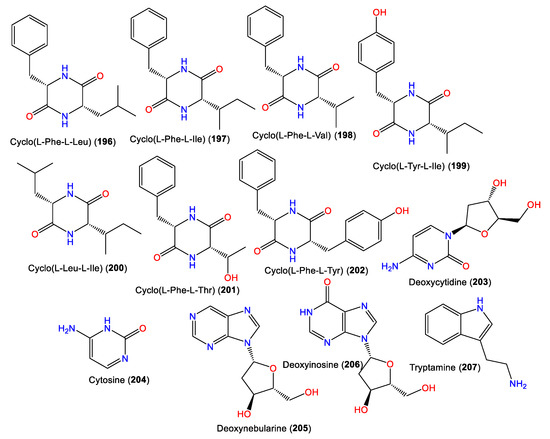
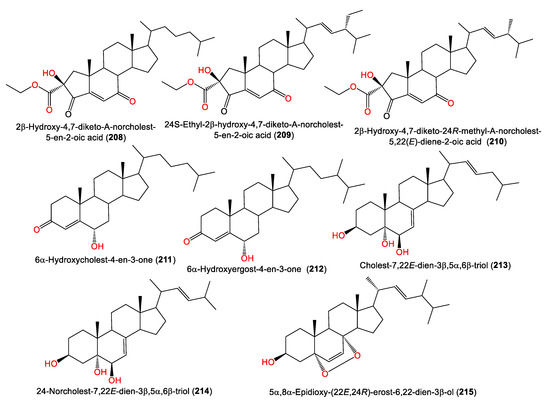
4. Steroid Compounds
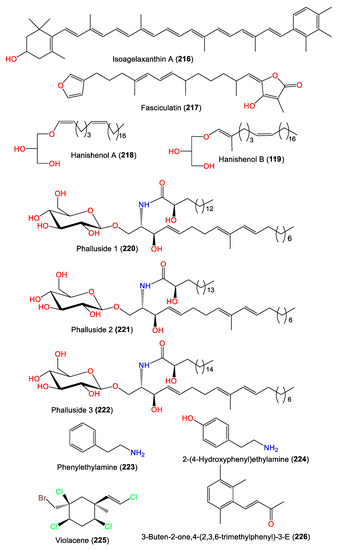
5. Other Metabolites
This entry is adapted from the peer-reviewed paper 10.3390/md21040257
