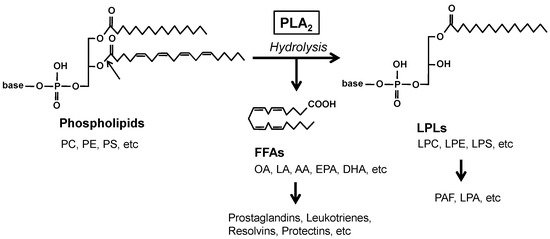
Phospholipase A
2 (PLA
2) enzymes hydrolyze the
sn-2 position of glycerophospholipids (hereafter phospholipids) to generate free fatty acids (FFAs) and lysophospholipids (LPLs) (
Figure 1). The mammalian genome encodes more than 50 PLA
2s or related enzymes, which are classified into several families based on their structures and functions [
1]. The PLA
2 reaction is important for the production of lipid mediators since polyunsaturated fatty acids (PUFAs) and LPLs released by PLA
2s can be converted to a wide variety of bioactive lipids referred to as lipid mediators, such as prostaglandins, leukotrienes, resolvins, and platelet-activating factors. In addition, PLA
2s can also be involved in membrane remodeling by altering phospholipid composition, energy production by supplying FFAs as β-oxidation substrates, or regulation of microenvironmental lipid milieu by fine-tuning the balance between saturated and unsaturated FFAs. Moreover, several PLA
2 enzymes catalyze non-PLA
2 reactions, such as phospholipase A
1, phospholipase B, lysophospholipase, triglyceride lipase, and transacylase reactions. As such, these hydrolytic actions of PLA
2s on a wide variety of lipids are associated with various pathophysiological events, ranging from the maintenance of tissue homeostasis to inflammatory, immunological, metabolic, cardiovascular, reproductive, neurodegenerative, and oncogenic disorders.
In contrast to intracellular PLA
2s, secreted PLA
2s (sPLA
2) are ideally positioned to cleave phospholipids available on the cell surface or in the extracellular milieu [
2]. The sPLA
2 family in mammals contains 11 members, namely IB, IIA, IIC (present in mice and rats, but pseudogene in humans), IID, IIE, IIF, III, V, X, XIIA, and XIIB (catalytically inactive), according to their sequence homology as well as the number and position of disulfide bonds [
2]. Historically, sPLA
2-IB and -IIA are two classical sPLA
2s originally identified by protein purification in the late 1980s. While sPLA
2-IB is secreted from the pancreas into the intestinal lumen and acts as a digestive enzyme [
3], sPLA
2-IIA, initially identified in the synovial fluid of arthritis patients and in platelets, is the only sPLA
2 that is abundantly detected in the circulation of patients with inflammation or infection and has been considered to participate in systemic or local inflammation and antibacterial defense [
4]. sPLA
2-IIC and -V were identified by genomic sequencing of the locus close to the sPLA
2-IIA (
Pla2g2a) gene in 1994 [
5,
6,
7,
8,
9]. Soon afterward, from 1997 to the early 2000s, sPLA
2-IID, -IIE, -IIF, -III, and -X, as well as two sPLA
2-XII isoforms, were identified using EST database searches [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. Group I/II/V/X sPLA
2s are structurally related, low-molecular-mass enzymes with a conserved His-Asp catalytic dyad, a Ca
2+-binding loop, and 6–8 disulfide bonds that ensure strict Ca
2+-dependent PLA
2 reaction and protein stability, while group III and XII sPLA
2s are each structurally atypical, having homology with group I/II/V/X sPLA
2s only in short stretches of the catalytic and Ca
2+-binding sites. Individual sPLA
2s exhibit unique tissue and cellular distributions and exert specific functions in a lipid mediator-dependent or, possibly, -independent manner. In general, individual sPLA
2s exert their specific functions within tissue microenvironments in which they are locally expressed. Although the activity of sPLA
2s on mammalian cells is relatively weak, they can act in a paracrine fashion on the plasma membrane of activated, damaged, or dying cells in preference to resting cells [
20,
21,
22]. More importantly, non-cellular phospholipid components, such as dietary lipids, lipoproteins, lung surfactants, extracellular vesicles (EVs), and membranes of invading microorganisms, such as bacteria and possibly fungi and parasites, act as excellent hydrolytic targets of sPLA
2s [
23,
24,
25,
26,
27,
28]. In some cases, sPLA
2-binding proteins such as PLA2R1 (sPLA
2 receptor) modulate the functions of sPLA
2 [
29,
30].
2. sPLA2 in the Epidermis
The epidermis is a highly organized stratified epithelium with distinctive keratinocyte layers. Notably, sPLA
2-IIF is the major sPLA
2 expressed in the suprabasal layers of mouse and human epidermis [
65]. Under the basal state,
Pla2g2f−/− mice exhibit only mild skin abnormalities, characterized by a fragile stratum corneum with modest perturbation of skin barrier function and acidity. These phenotypes are more pronounced in the abdominal skin of an adult, but not neonatal,
Pla2g2f−/− mice, suggesting that although sPLA
2-IIF is not a major player in the central program of epidermal differentiation, it contributes to increasing stratum corneum stability against environmental stresses such as friction against the floor or prolonged exposure to skin microbiota. After tape-stripping of the stratum corneum,
Pla2g2f−/− mice display delayed recovery from the skin barrier damage, suggesting that sPLA
2-IIF accelerates epidermal repair [
60]. The impact of sPLA
2-IIF ablation is more dramatic in primary keratinocytes in culture, where the cells fail to be properly differentiated and activated when sPLA
2-IIF is genetically ablated or pharmacologically inactivated by a pan-sPLA
2 inhibitor [
65]. Thus, loss of sPLA
2-IIF impairs proper keratinocyte differentiation and barrier formation in mice.
Global or skin-specific transgenic mice overexpressing mouse sPLA
2-IIF (
Pla2g2f-TG) spontaneously develop psoriasis-like epidermal hyperplasia and alopecia, with increased expression of a panel of psoriasis markers, including S100A9 and IL-36α [
65]. Moreover, sPLA
2-IIF is induced in mouse skin treated with imiquimod, an inducer of experimental psoriasis, and is also highly expressed in the hyperplasic epidermis of patients with psoriasis. Importantly, genetic deletion of sPLA
2-IIF in mice protects against epidermal hyperplasia and associated inflammation in models of Th17-dependent psoriasis, Th1-dependent contact hypersensitivity (CHS), and skin carcinogenesis. These findings indicate that sPLA
2-IIF is associated with the exacerbation of epidermal-hyperplasic diseases. Mechanistically, sPLA
2-IIF preferentially hydrolyzes plasmalogen (alkenyl-type phosphatidylethanolamine (PE)) having an
sn-2 PUFA (docosahexaenoic acid (DHA) in particular) secreted from keratinocytes to yield lysoplasmalogen (P-LPE). This unique LPL facilitates aberrant proliferation and activation of keratinocytes, leading to the propagation of skin inflammation. Indeed, the levels of P-LPE in mouse skin are correlated well with the expression levels of sPLA
2-IIF in multiple skin disease models, and topical application of P-LPE to
Pla2g2f−/− skin in vivo or supplementation of
Pla2g2f−/− keratinocytes with P-LPE ex vivo restores the psoriasis-related phenotypes [
65].
Thus, the sPLA2-IIF/P-LPE axis has beneficial and detrimental roles in skin barrier formation and epidermal-hyperplasic inflammation, respectively, thereby regulating the physiology and pathology of the skin. Since LPLs with an sn-1 alkenyl moiety are structurally unstable and can be readily degraded non-enzymatically under acidic conditions, it is plausible that P-LPE exists more stably in inflamed skin where epidermal pH becomes close to neutral. In contrast, in healthy skin where epidermal pH is mildly acidic, P-LPE might be further converted to a certain stable metabolite that regulates skin homeostasis and repair.
3. sPLA2 in Hair Follicles
Abnormalities in skin lipid metabolism vary and often severely affect hair cycling, causing hair loss or alopecia [
49,
55]. Hair follicles in the skin undergo repeated cycles of growth (anagen), regression (catagen), and rest (telogen) during life [
68]. sPLA
2-IIE is abundantly expressed in hair follicles during the anagen period, being distributed in companion cells of the outer root sheath and cuticular cells of the inner root sheath [
66]. In
Pla2g2e−/− mice, hair follicles show a detachment between the follicular epithelium (cuticle) and hair shaft and altered expression of some hair follicle-related genes, but with little or no abnormalities in the epidermis. Lipidomics analysis has revealed that sPLA
2-IIE mobilizes various unsaturated FFAs and LPE species in mouse skin, consistent with the in vitro substrate specificity of sPLA
2-IIE. However, it remains unclear which lipid metabolites mobilized by sPLA
2-IIE participate in hair follicle homeostasis and whether sPLA
2-IIE also plays a similar role in hair quality control in human skin.
4. sPLA2 in Lymphoid Tissues That Affects Skin Diseases by Regulating Adaptive Immune Responses
While sPLA
2-IIE and sPLA
2-IIF are abundantly expressed in keratinocytes of the upper epidermis and hair follicles, respectively (see above), sPLA
2-IID is barely detectable in mouse skin. Instead, sPLA
2-IID is expressed abundantly in dendritic cells (DCs) and macrophages, especially CD4
+CD11b
+CD11c
+ MHC class II
lo DCs and M2-like macrophages, in secondary lymphoid organs such as the spleen and lymph nodes (LNs) of mice and humans [
69,
70]. Furthermore, sPLA
2-IID expression is downregulated, rather than upregulated, in DCs stimulated with antigen or lipopolysaccharide [
69,
71]. A lipidomics-based PLA
2 enzyme assay using a natural phospholipid mixture extracted from mouse lymphoid tissue as a substrate [
72] indicates that sPLA
2-IID preferentially hydrolyzes PE species with an
sn-2 PUFA, including ω6 AA and, more efficiently, ω3 eicosapentaenoic acid (EPA) and DHA, rather than those with oleic acid or linoleic acid. This enzymatic preference of sPLA
2-IID for PE species with an ω3 PUFA as substrates, along with its distribution in lymphoid immune cells and downregulation by proinflammatory stimuli, suggests that sPLA
2-IID has a resolving, rather than promoting, role in the adaptive immune response. In fact, despite the low expression of sPLA
2-IID in the skin,
Pla2g2d deficiency leads to exacerbation of CHS and psoriasis, likely because sPLA
2-IID attenuates adaptive immunity in the LNs, thereby sequestering pathogenic Th1 and Th17 immune responses [
69,
70].
In a model of Th1-dependent CHS, topical application of the hapten antigen dinitrofluorobenzene to abdominal skin (sensitization), followed by a second application of the same antigen to ear skin (elicitation), induces ear swelling. In the elicitation phase of CHS, the resolution of inflammation in the skin and LNs is delayed in
Pla2g2d−/− mice [
69]. In this state, expression levels of the Th1 cytokines IFN-γ and IL-12 are greater in the draining LNs of
Pla2g2d−/− mice than in those of littermate wild-type (WT) mice. Likewise, in a model of psoriasis,
Pla2g2d−/− mice display more severe epidermal hyperplasia than do WT mice, with increased IL-17A
+ or IL-22
+ T cells in the affected skin and LNs [
70]. Furthermore, DCs isolated from
Pla2g2d−/− mice are hyperactivated even without stimulation. Mechanistically, sPLA
2-IID in the LNs constitutively hydrolyzes PUFA-containing PE species (possibly in EV membranes) to mobilize ω3 PUFA-derived anti-inflammatory lipid mediators that put a brake on DC-mediated adaptive immunity. Indeed, steady-state levels of ω3 PUFAs and their metabolites, such as DHA-derived resolvin D1 (RvD1), are markedly reduced in LNs from
Pla2g2d−/− mice compared to WT mice. Conversely,
Pla2g2d-TG mice display milder inflammation than do WT mice in the CHS and psoriasis models, with increased levels of ω3 PUFA metabolites [
70]. ω3 PUFA-derived resolvins and maresins suppress acquired immunity by attenuating DC migration, activation, and antigen presentation to T cells and by preventing IgE class switching in B cells [
69,
73,
74,
75]. Moreover, these ω3 PUFA-derived lipid mediators can facilitate the polarization of anti-inflammatory M2 macrophages [
76,
77], consistent with the fact that fewer M2 macrophages are present in the LNs of
Pla2g2d−/− mice [
70].
On the other hand, the beneficial role of sPLA
2-IID in counteracting pathogenic Th1/Th17 immune responses can be conversely disadvantageous in some situations, such as host defense against infection and cancer [
70,
78]. Indeed, sPLA
2-IID promotes, rather than prevents, the development of skin tumors, likely because it attenuates anti-tumor Th1 immunity. Accordingly,
Pla2g2d−/− mice are protected against skin carcinogenesis, with increased tumor-suppressing IFN-γ
+CD8
+ cytotoxic T cells and M1 macrophages [
70]. Thus, the immunosuppressive function of sPLA
2-IID provides “good” or “bad” outcomes in distinct disease settings, ameliorating skin inflammation and exacerbating skin cancer. sPLA
2-IID also alleviates anti-viral immunity, possibly through mobilizing anti-inflammatory PGD
2 in the lung, and ultimately exacerbates coronavirus-induced acute lung injury [
78,
79,
80]. Thus, specific inhibition of sPLA
2-IID in patients with certain types of cancer or infection could be an attractive therapeutic intervention for restoring immunological functions, a concept reminiscent of “immune checkpoint” therapy.
5. sPLA2 Involved in an Alteration of the Intestinal Microbiota That Secondarily Affects Skin Diseases
Since sPLA
2-IIA is induced in various tissues during inflammation in humans and rats, its functions have been proposed to be related to the exacerbation of inflammation through the production of lipid mediators at the local sites of expression and to host defense against infectious bacteria through the degradation of bacterial membrane phospholipids [
4]. However, the
Pla2g2a gene is naturally disrupted in C57BL/6 and 129/Sv strains due to a frameshift mutation [
81], which makes it difficult to assess the precise functions of endogenous sPLA
2-IIA in vivo using a standard knockout strategy. Other mouse strains, such as BALB/c, C3H, and DBA/1, have an intact
Pla2g2a gene [
81], but unlike the situation in humans and rats, its expression in these mouse strains is highly restricted to the intestine [
82,
83]. Despite this biased distribution, genetic deletion of sPLA
2-IIA in the BALB/c strain results in attenuation of the development of carcinogen-induced skin cancer and aggravation of imiquimod-induced psoriasis [
84]. Therefore, it seems a mystery why these phenotypes are manifested in mouse skin where sPLA
2-IIA is minimally expressed.
In the small intestine, sPLA
2-IIA is predominantly expressed in Paneth cells that secrete a variety of antimicrobial peptides, and its expression is significantly reduced by antibiotic administration [
84]. This raises the possibility that sPLA
2-IIA may be induced by intestinal bacterial components and, as an antimicrobial protein, may have a secondary effect on the skin by degrading intestinal bacterial membranes and thereby altering the balance of the gut microbiota. A comparative analysis of the gut microbiota has revealed apparent differences in several bacterial genera (
Helicobacter,
Ruminococcus,
Lachnospira, etc.) between
Pla2g2a−/− and WT mice. Furthermore, when
Pla2g2a−/− mice and WT mice are co-housed from birth, under which gut microbiota in the two groups no longer differ, the differences in skin phenotypes between the genotypes are lost. The small intestine of
Pla2g2a−/− mice shows notable changes in the expression of a group of genes involved in the epithelial barrier and immunity, especially that of immunoglobulin genes, reflecting the differences in the intestinal microbiota. Various plasma metabolites, including those involved in immune modulation and oncogenesis, are significantly altered in
Pla2g2a−/− mice compared to WT mice. A comprehensive analysis of fecal lipids has revealed a significant decrease in unique lipid metabolites that are likely to be derived from the gut bacteria rather than from the host in
Pla2g2a−/− mice. Furthermore, when housed in a cleaner animal facility, the intestinal microbiota, including
Helicobacter and
Ruminococcus, are reduced, the intestinal expression of sPLA
2-IIA is decreased, and the skin phenotypes caused by sPLA
2-IIA deficiency are mitigated. These results collectively suggest that sPLA
2-IIA secreted from small intestinal Paneth cells is involved in the shaping of the intestinal microbiota and that when this pathway is perturbed, the intestinal microbiota are altered, blood metabolites and immune responses are changed, and the phenotypes eventually become evident in distal organs such as the skin. In further support of this conclusion, PLA2G2A-TG mice on the C57BL/6 strain also display an alteration in the gut microbiota, which leads to the exacerbation of systemic inflammation and arthritis [
85].
These findings are the first to elucidate the function of sPLA2-IIA in the intestinal tract, which has remained unknown for many years. Since several sPLA2 isoforms other than sPLA2-IIA are also expressed in the intestinal tract, it is possible that they may also affect the pathophysiology in distant organs via regulation of the intestinal microbiota, although future work is needed to prove this hypothesis and generalize the theory. Beyond the difference in the expression profiles of sPLA2-IIA between humans and mice, as mentioned above, high expression of sPLA2-IIA in the intestine of both species suggests that this bactericidal sPLA2 is likely to be involved in the regulation of the intestinal microbiota in humans as well. Therefore, drug discovery targeting sPLA2-IIA in the intestinal tract may be useful for new diagnoses and treatment of skin diseases.
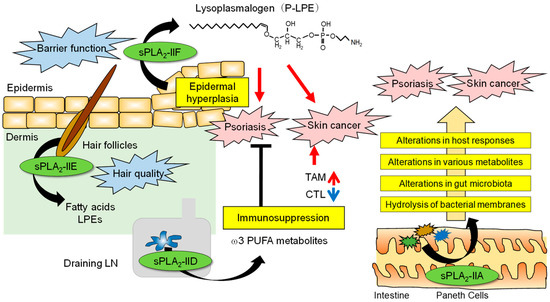
The roles of sPLA2s in skin homeostasis and disease are summarized in Figure 2.
Figure 2. The roles of sPLA2s in skin homeostasis and disease. The skin is an organ that interfaces between the host and the external environment. The major sPLA2s expressed endogenously in mouse skin are sPLA2-IIF and sPLA2-IIE, the former in the epidermis and the latter in hair follicles, depending on the hair cycle. Following a psoriatic stimulus, sPLA2-IIF is induced in epidermal keratinocytes by Th17 cytokines and preferentially hydrolyzes plasmalogen to give rise to P-LPE, which in turn promotes epidermal hyperplasia and inflammation. In contrast, sPLA2-IID blocks Th17 immunity in the draining LN through the production of ω3 PUFA metabolites, thereby putting a break on psoriasis. In the case of skin cancer, P-LPE produced by epidermal sPLA2-IIF promotes hypergrowth of skin cancer without affecting its incidence, while ω3 PUFA metabolites produced by sPLA2-IID in the LN decrease IFN-γ+CD8+ cytotoxic T cells (CTLs) and increase M2-like tumor-associated macrophages (TAMs). As such, sPLA2-IID reduces anti-tumor immunity and ultimately facilitates tumor formation and growth. In the intestinal lumen, sPLA2-IIA secreted from Paneth cells acts as an antimicrobial protein to shape the gut microbiota, thereby secondarily affecting host responses, including psoriasis and skin cancer.