Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan that has a well-known ability to bind with the cluster of differentiation 44 (CD44) receptor on classically activated M1 macrophages. HA-nanosystems can be used for targeted drug delivery to counter inflammation and, subsequently, pain.
- hyaluronic acid
- CD44
- nanosystems
- antinociceptive
1. Introduction
In response to environmental factors and noxious stimuli, the body uses pain as a defence mechanism. Pain is a proactive beneficial immune response in the acute phase, but neuropathic pain becomes problematic in the chronic phase. The nociceptive sensory neurons (nociceptors) activate the neuropathic pain signal, but the immune system also plays a significant role in defining the active bidirectional crosstalk between pain and inflammation [1]. Nociceptors can control innate and adaptive immune functions by releasing neuropeptides and neurotransmitters [2][3][4][5]. In response, neuronal plasticity and chronic pain can be controlled by mediators (lipids, cytokines, and growth factors) released by the immune cells [6][7][8]. The signals and messages sent by the nervous system are propagated in milliseconds. This is theorised to be partly why nociceptors are ideally positioned to be first responders to pathogens and tissue injury. Nociceptors release neuropeptides in adverse situations that activate the macrophages of the immune system to control neuropathic pain and inflammation. Increasing evidence from studies shows that macrophages can induce and resolve pain via macrophage–nociceptor interactions [9][10][11].
Inflammation is a complicated process. It was previously known to be a response to infection; however, in recent years, inflammation was found to cause multiple diseases: atherosclerosis [12], depression [13], Alzheimer’s [14], obesity [15], etc. [16][17]. The elevation of inflammatory markers (C-reactive protein) or release of pro-inflammatory cytokines can be detected to confirm its presence [18]. Even a minimal increase in the expression of these markers increases the risk of inflammation, which is abnormal without an externally harmful stimulus. An unhealthy lifestyle can be a cause of abnormal inflammation [19][20][21].
2. HA and Inflammation: Influence of Molecular Weight
Different molecular weights (MW) of HA are present in all biological tissues and fluids [22] (Figure 1). Indigenously, high-molecular-weight (HMW) HA is found, which is then degraded into smaller fragments of low molecular weight (LMW) depending on the environmental factors [23][24]. This degradation of HA is essential for a number of bodily functions, for instance, as a lubricant in the synovial fluid [23][25].
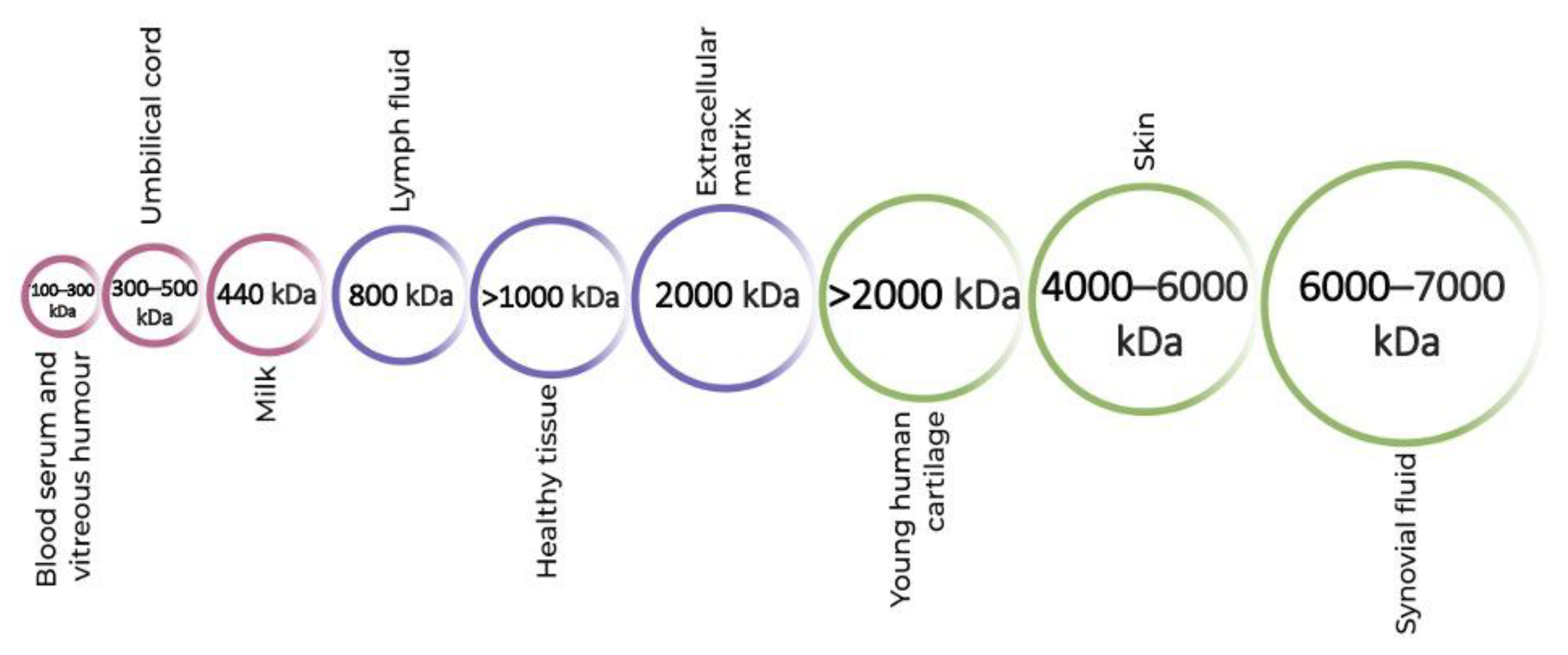
Figure 1. Molecular weights of hyaluronic acid in different parts of the human body.
The manipulation of MW of HA in drug delivery systems can lead to analgesic, anti-inflammatory and immunostimulatory results [23][24][25][26]. Although there is a lack of detailed research on the specificity of the antinociceptive effects of HA of different MW, it is widely accepted that HMW HA inhibits the activation of lipopolysaccharide (LPS) by directly binding to the toll-like receptor-4 (TLR-4) under inflammatory conditions [27].
To maintain proper functioning, there needs to be a balance between the quantity of HA being produced and degraded in the body [23]. The MW of HA is controlled through the body by shifting between its cellular uptake and degradation under homeostatic conditions. The enzymes hyaluronidases control the degradation of HA [28][29]. Two types of hyaluronidases (HYAL), HYAL1 and HYAL2, are involved in the active degradation of HA. While HYAL1 targets LMW HA, HYAL2 is known for breaking down HMW HA chains to 20 kDa [28].
There is a long-running debate in the research regarding the pro/anti-inflammatory properties of HAs of different molecular weights. Studies, including that of Isa et al., have suggested that HMW HA is shown to have anti-inflammatory properties by inhibiting the production of interleukin-1β, one of the more prominent inflammatory cytokines, and that LMW HA is a promoter of inflammation [30][31][32][33][34][35]. Baeva et al. provided the explanation that the breakdown of longer chains of HMW HA by HYAL2 produces HAs with fewer disaccharides of LMW HA that accumulate at inflamed sites to activate the nuclear factor kappa-light-chain-enhancer of the activated B cells (NF-κB) pathway [33] (Figure 2). However, results from the HA hydrogel osteoarthritis (OA) therapy study by Agas et al. showed that LMW HA (37,900 Da) promotes anti-inflammatory properties. The LMW HA hydrogel significantly lowered the expressions of pro-inflammatory cytokines, tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-1 [34]. Chernos et al. performed anti-inflammatory studies on human cell lines with butyrylated derivatives of LMW HA to provide optimal visco-supplements for OA therapy [31]. Chistyakov et al. also noted that long-term exposure to LMW HA can suppress the inflammation induced by the TNF-α pathway [36]. Inflammation is a complex mechanism that involves two major human systems, the nervous system and the immune system. Ongoing research is attempting to unravel the mysteries that surround the molecular weights of HA and their effect on pain and inflammation; however, there is no sure way to say which molecular weight causes, and which resolves, inflammation at present.
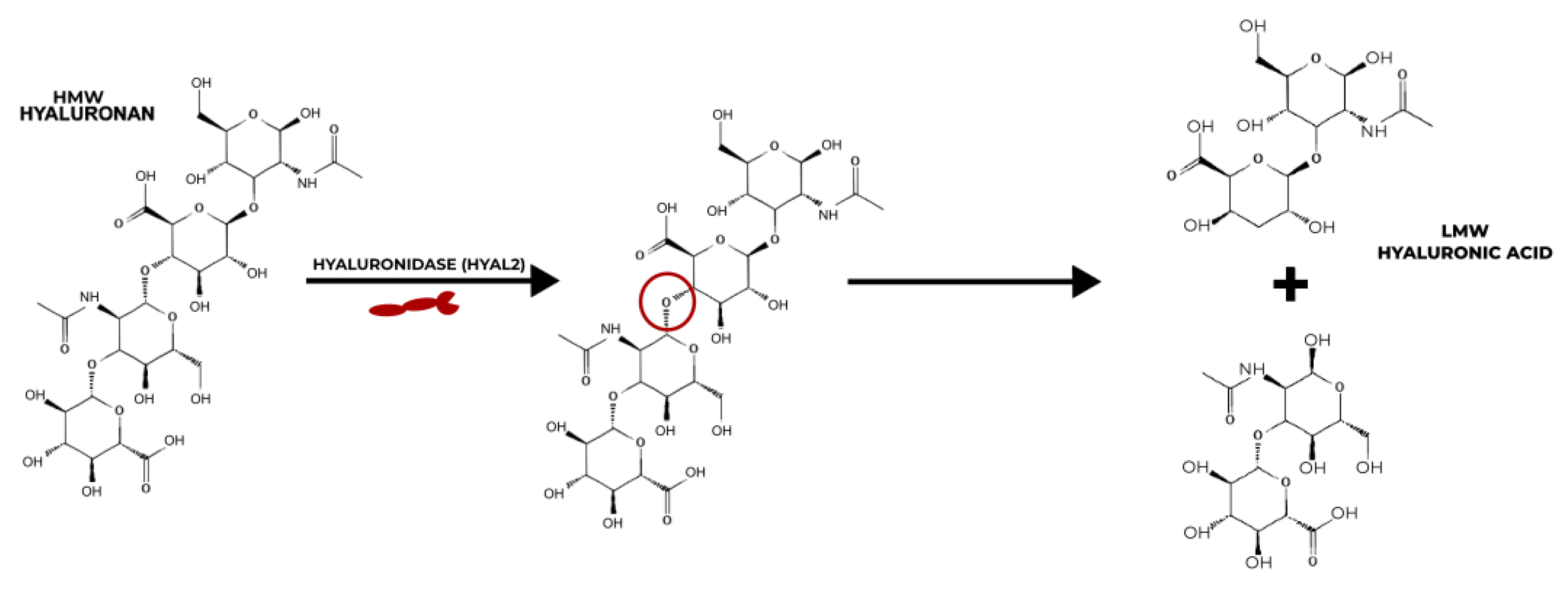
Figure 2. Mechanism of action of HYAL2 on the degradation of HMW HA to LMW HA.
3. HA-Based Nanosystems
HA-based nanosystems can be categorised in a number of ways, such as nanohydrogels, nanoparticles and self-assembling nanosystems (Figure 3). A variety of delivery system designs have been considered for this entry that display the vast potential of using HA to counter inflammation, which is notoriously at the root of many chronic diseases.
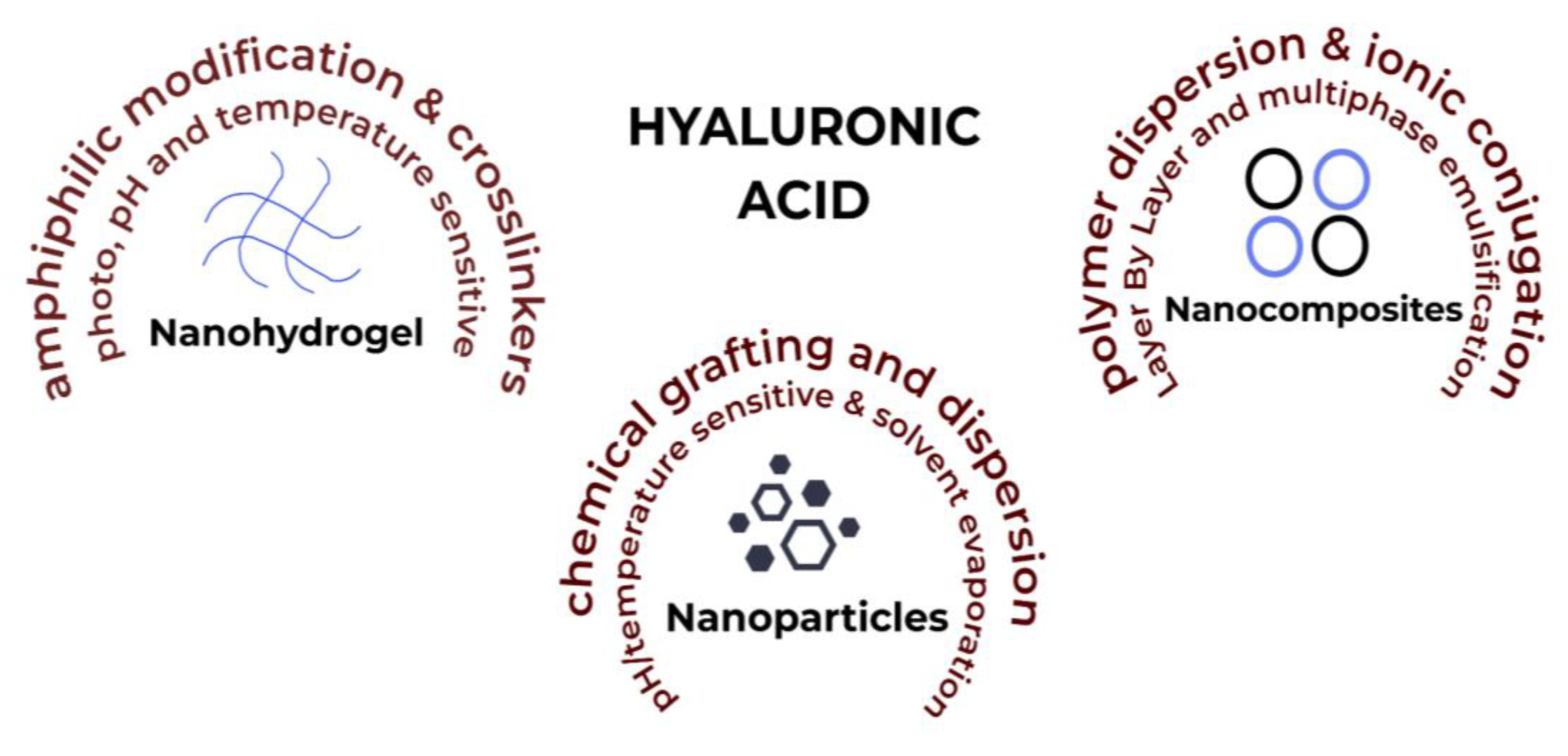
Figure 3. HA-based nanosystems with factors affecting their synthesis and production.
3.1. Drug Delivery Systems
Drug delivery systems describe how the drugs are carried into and throughout the body. The following studies use HA to load drugs that are further tested in vitro and/or in vivo.
Nanohydrogels are three-dimensional (3D)-polymeric networks of nanoscale dimensions, with a crosslinked structure that gives them potential flexibility and versatile behaviour [37][38]. They have the dual advantage of hydrogels, allowing for the high encapsulation efficiency of hydrophilic compounds, and of nanostructures, allowing for high cellular internalisation [39]. Environmental stimuli such as temperature and pH can be used to develop site-specific nanoparticles which makes them an optimal choice for novel theranostic applications [40][41][42].
Quagliariello et al. synthesized a quercetin-loaded HA nanogel for its anti-inflammatory effect in breast tumour cells [43]. The 200 kDa HA that was used provided protection to the drug from oxidative and enzymatic degradation in the tumour environment. A solvent–non-solvent method was used for synthesis, with glutaraldehyde as a crosslinker. The drug-loaded nanogel showed a size of 211 nm. Free HA was noted as having insignificant cytotoxicity; however, the crosslinker in the nanogel showed a 10–20% cytotoxic effect. The expressions of anti-inflammatory cytokines (IL-8, IL-6 and IL-19) decreased by up to 55% with nanogel when compared to the control. A 30–40% increase in anti-oxidative effect was observed when quercetin was co-loaded with everolimus. The group concluded that HA-nanohydrogels provide an excellent template for studying tumour microenvironments, opening perspectives for further studies.
Barbarisi et al. tested the effect of co-loading quercetin and temozolomide in HA-nanogel as a therapy with an anti-inflammatory effect in glioblastoma tumour cells [44]. The solvent–non solvent method was used to make nanogel with 200 kDa HA and a glutaraldehyde crosslinker. The drug-loaded nanogel had a size of 197 nm and a ζ-potential of −31.3 mV. Just like in their previous study [43], the group noted cytotoxic effects from the crosslinker in the nanogel in this research. HA-induced, receptor-mediated endocytosis was noted with 30% nanogel internalization after 2 h. HA on the surface was used for this nanosystem to avoid opsonization by the RES and provide a longer drug retention time.
While both of the above-mentioned studies [43][44] successfully synthesized anti-inflammation-promoting nanogels, it should be noted that the glutaraldehyde used to stabilize these nanogels was responsible for increasing their cytotoxicity. Further studies need to be performed, which can either suggest a less toxic crosslinker or a formulation method that does not require a crosslinker for stabilization.
Storozhylova et al. were looking for an efficient drug delivery system that showed a longer retention rate for the treatment of inflammatory joint diseases [45]. The group synthesised in situ forming, non-crosslinked HA-fibrin hydrogels, containing HA-nanocapsules co-loaded with dexamethasone and galectin-3 inhibitor. The drug-loaded nanocapsules showed the suppression of inflammation after intra-articular administration. However, the study noted that further investigation was required to treat chronic synovial inflammation.
It is beneficial to develop a drug delivery system with positive results in terms of efficiency, but it is even better to design a system with a hassle-free administration route. Even though injectable nanohydrogels are considered non-invasive techniques, transdermal drug deliveries take this definition one step further. As previously mentioned, transdermal drug delivery (TDD) has classically more successfully been associated with the use of nanoparticles. TDD is a painless method of delivering therapeutics onto intact skin [46][47]. The nanosize [48], drug retention [49], and drug release rates [50] of polymeric nanohydrogels are winning factors in their wide use as a targeted TDD method, and nanohydrogel size is essential for successful skin penetration. Nanohydrogels can be manipulated into loading drug-loaded nanocapsules that successfully penetrate the skin and intake water. This leads to the swelling of the nanocapsules and subsequent drug release [51][52][53].
Wei et al. tested the anti-inflammatory effect of topically administered HA nanohydrogels with baicalin–nanocrystals (NC) [54]. An 800−100 kDa HA nanogel was used to assist the skin permeability of poorly soluble baicalin. Four w/v concentrations of HA were used to optimise the nanogel: 0.5%, 1%, 1.5% and 2%. The increase in HA concentration led to an increase in the viscosity and elasticity of the nanogel; however, it also saw a decrease in the drug release rate and skin permeation rate. The 1% w/v HA was chosen as the optimal concentration, with a 20-fold increase in skin permeability as compared to the control. The size of 1% w/v HA nanogel was 193 nm.
The biodegradability of HA allows for homogenous drug distribution in the gel matrix [55][56]. Liu et al. used electrospinning to make an absorbable nanofibrous hydrogel for wound healing under chronic diabetic conditions [57]. A 1400 kDa thioether grafted HA, crosslinked with Fe3+ (FHHA-S/Fe) nanogel, was synthesized for the purpose of wound healing by modulating the site of injury. Overall, the crosslinking increased the stability of the nanofibres by two-fold. Complete absorption of the nanogel was observed at 72 h. The thioether grafting increased IL-4 expression, leading to 33% and 18% faster wound healing as compared to the non-ether nanogel. A 24% decrease in the expression of M1 macrophages was observed, along with a 22% increase in the expression of M2 macrophages after treatment with HMW HA nanogel.
Pleguezuelos-Villa et al. synthesized mangiferin-loaded HA-based nanoemulsions for their anti-inflammatory effect on skin lesions [58]. Two ranges of the MW of HA were tested for the nanoemulsions, 40–50 kDa (LMW) and 1000–1200 kDa (HMW). The LMW HA nanoemulsion size was detected at 221 nm, and for HMW it was 393 nm. A gradual, sustained release of the drug was found in all nanosystems after 24 h but LMW HA nanoemulsion with surfactant was the highest, with 10–15%. The different MW of HA did not affect the oedema inhibition for the anti-inflammatory activity of the nanoemulsion. However, the use of a surfactant decreased the oedema inhibition activity of the nanoemulsion. HMW HA only seemed to affect the size of the nanoemulsion.
Manca et al. used curcumin-loaded HA vesicles (hyalurosomes) for skin lesions [59]. This study evolved the field of vesicles by synthesizing hyalurosomes. The group enhanced the properties of conventional liquid vesicles by including a gel-core structure to provide more stability and a nanosized diameter to make them nanovesicles. The organic solvent-free polymer dispersion method was used for the synthesis. Two w/v concentrations of 200–400 kDa HA were tested: 0.1% and 0.5%. The concentration of 0.1% showed a size of 166 nm and an encapsulation efficiency of 76%, whereas that of 0.5% showed a size of 157 nm and a curcumin encapsulation efficiency of 79%. The enhanced nanovesicle structure increased the encapsulation by 10–13% as compared to conventional liposomal vesicles. However, 0.5% w/v hyalurosomes showed a higher viscosity than 0.1% and more stiffness, by 14%, with the addition of curcumin. An increased improvement in induced skin lesions in in vivo tests was seen in 0.5% w/v hyalurosomes. Re-epithelialized skin was also observed by day 6.
Yang et al. synthesized HA nanostructured lipid carriers (NLCs) loaded with ropivacaine (RVC) and dexmedetomidine (DMDT) [60]. NLCs are evolved lipid nanoparticles that include both liquid and solid lipids that decrease the order of crystal arrangement of conventional lipid nanoparticles to provide a higher drug loading efficiency [61]. The group used the solvent diffusion method to create an HA-based (3 kDa) drug delivery system that increased the duration of the analgesic effects of RVC and DMDT by 75%. The NLCs had a size of 108 nm, a ζ-potential of −30.7 mV due to the presence of HA, and drug encapsulation efficiency of 89.5% for RVC and 88.1% for DMDT. The HA NLCs also increased the cell viability of the drugs from 61.2% (free drug solution) to 80%. The group also performed in vivo skin permeation tests that showed that the encapsulation of the drugs in the NLCs increased their permeability by 67%. The NLCs also increased the antinociceptive effect of the drugs by 80 min. It was found that co-loading RVC and DMDT had a higher analgesic effect than loading a single drug.
Yue et al. also decided to formulate NLCs for TDD of their drug bupivacaine (BPV) for local anaesthesia [62]. The group used 300 kDa HA modified with linoleic acid and polyethylene glycol (PEG) for stealth properties. The NLCs were made with lipid melt-emulsification and solvent injection techniques that had a size of 154 nm, a ζ-potential of −40.1 mV and a BPV encapsulation efficiency of 88.9%. The NLCs showed a cell viability of 70% as compared to the 40% obtained by free drug solution. In vivo tests showed an increased antinociceptive effect by the NLCs as compared to free drug solution, by 50%.
Both the above-mentioned studies [60][62] used NLCs for their formulations; however, there were some notable differences between the two drug delivery systems. After 75 h of administration, one of the NLCs [60] showed a 70% antinociceptive activity, whereas this was only at 60% for the other NLC [62]. While this may not be a major difference, it is possible that the synergistic effect of co-loading two drugs favoured the former. The higher NLC size used by the latter can be attributed to their use of HMW HA.
Iannitti et al. tested the efficacy of an HA- and chondroitin sulfate (CS)-based medical device called Esoxx® for the treatment of inflammation of the gastric mucosa, also known as gastritis [63]. The presence of HA and CS was seen to reduce inflammation and the discomfort that comes along with it in the tested patients. HA provides hydrophilicity to the submucosal connective tissue, which provides it with a better chance of healing. The group, however, concluded that further studies are needed, with a higher number of patients, for definitive results.
3.2. Macrophage Targeting Nanosystems
It has been well established that macrophages have an important role to play in modulating inflammatory responses and pain [10][11][64]. The pro-inflammatory M1-macrophage phenotype is responsible for the first line of defence, which is inflammation. Hence, for antinociceptive therapies, studies have developed nanosystems that target the CD44 receptor, which is heavily present on the surface of M1 macrophages. These therapies either work by lowering/inhibiting the effect of pro-inflammatory cytokines or polarising the macrophage phenotype from M1 to M2.
Zhang et al. used a layer-by-layer (LBL) NLC system for lidocaine (LA)-loaded chitosan and HA drug delivery systems [65]. LBL involves the alternative deposition of oppositely charged polyelectrolytes via electrostatic interaction for the assembly of multilayer films. This method decreases the drug release rate and enhances skin permeability. The group compared the characteristics of LBL-NLCs with simple NLCs. It is important to note that the size of the nanoparticles is a major deciding factor in whether the drug delivery system will reach the target site. The NLCs showed a size of 181 nm and a ζ-potential of +37.6 mV due to the outermost layer of chitosan. The in vivo tests for anaesthesia showed that NLCs had a 30% effect after 24 h of administration, whereas LBL-NLCs had an 80% effect. The combined biocompatibility of hyaluronic acid and chitosan provides a great template for the loading of drugs and target studies [66].
Farajzadeh et al. synthesized curcumin-loaded HA-polylactide (PLA) nanoparticles to test CD44-targeted antinociceptive activity and macrophage repolarization [64]. An HA of 20 kDa MW was used to prepare HA-PLA conjugates of 102 nm in size, with a ζ-potential of -24.5 mV and a curcumin encapsulation efficiency of 88%. Mouse peritoneal macrophages were used for in vitro studies that detected a burst release in the first 8 h of administration and a consistent subsequent release with 33% drug release after 144 h (6 days). The initial burst release could be due to the presence of the drug, which was closer to the surface of the nanoparticles. In vivo nanoparticle uptake was promoted by endocytosis; hence, it was necessary to stimulate the nanoparticles under similar conditions. Endocytosis is supported by an acidic environment, so the group also tested the drug release from the nanoparticles at a pH of 4.4. This test showed a 50% drug release after 120 h of administration. Markers for M1 and M2 macrophages (iNOS and Arg-1, respectively) were quantified to check macrophage repolarization. Curcumin-loaded nanoparticles increased Arg-1 expression by 50% as compared to free drug administration. This concluded the successful repolarization from M1 macrophages to M2 macrophages. The expressions of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6, were also reduced by 86%, 85% and 87%, respectively, when compared to free drug administration.
Tran et al. also experimented with shifting the macrophage polarity as a means of therapy for inflammation [67]. However, instead of curcumin, they encapsulated plasmid deoxyribonucleic acid (pDNA) in their HA nanoparticles by modifying HA with a positively charged polymer poly(ethyleneimine) (PEI). The pDNA expressed interleukin-4 (IL-4) and interleukin-10 (IL-10) genes that inhibit the production of TNF-α, which is a direct promoter of M1-expressed inflammatory cytokines. This reduction in the expression of TNF-α also leads to lower M1 polarisation and subsequently higher M2 polarisation [68]. However, the direct administration of IL-4 and IL-10 is reported to have toxic effects. Therefore, the group combined gene therapy with nanotechnology to synthesise a nanosystem that not only represses inflammatory cytokines but also polarises macrophages towards alternative activation.
Kosovrasti et al. targeted another M1-specific cytokine to reduce inflammation, TNF-α [69]. The group encapsulated TNF-α-specific, small, interfering RNA (siTNF-α) in HA nanoparticles. The formulation of the nanoparticles involved the blending of HA-PEI, HA-hexyl fatty acid and HA-PEG. The 78–90 nm diameter HA nanoparticles encapsulating siTNF-α reduced the level of production of LPS-induced TNF-α in macrophages, and hence reduced inflammation. The group concluded that this study could be beneficial when researching therapies for acute inflammatory diseases.
It is important to note that the nanosystems developed in two previously mentioned studies [64][67] were not tested in vivo, and both groups concluded that further studies were needed to ascertain the effect of their respective HA-based nanosystems under complex in vivo homeostatic conditions. However, the HA nanoparticles encapsulating siTNF-α by the group [69] were tested in vivo, and the expected anti-inflammatory results were found. However, they also noted that the results from one study should not be considered conclusive evidence.
Xie et al. used HA-containing ethosomes (ES) for the delivery of rhodamine B for TDD [70]. Ethosomes are a kind of liposomal vesicles that are known for increasing skin permeability, drug accumulation and targeting drug delivery [71][72][73] to avoid systemic toxicity. A 150 kDa HA was used to enhance drug entrapment in the ES using amphiphilic modifications to the HA backbone. Different ratios of HA:ES were tested for optimization: 2.5:1, 5:1 and 10:1. The increase in the ratio of HA led to an increase in the size of the drug delivery system, with a range of 593–916 nm according to dynamic light scattering (DLS); however, transmission electron microscopy (TEM) detected the size of the system to be <100 nm. This difference could be because TEM analysis is conducted on a dry sample and DLS is performed in water, which leads to swelling of the particles. A high HA concentration also led to an increase in the quantity of the encapsulated drug. In vivo tests were conducted, which noted skin permeation within 30 min of administration. HA was also noted to increase the effectiveness of the system by 30% compared to the drug delivery system without HA. Therefore, the 5:1 ratio was concluded to be optimal.
The specific targeting ability of HA and its transdermal absorption is paramount in its use of TDD [74]. HA is a major synovial fluid component and artificially administered HA provides temporary relief for OA [75]. Zerrillo et al. decided to take advantage of the low pH conditions in the synovial fluid and synthesize HA-loaded, pH-responsive poly(lactic-co-glycolic acid) (PLGA) nanoparticles with a triggered burst release for OA [76]. The therapeutic approach of HA on OA partly includes reducing the inflammation at the OA site. The burst release of drug in this study was triggered by the ammonium bicarbonate loaded in the PLGA nanoparticles. The 750–1000 kDa HA was injected into the PLGA nanoparticles that showed a size of 202 nm and 28% encapsulation efficiency. In vitro studies showed that PLGA-HA nanoparticles had a faster uptake than only PLGA nanoparticles. In vivo studies were also conducted, which showed that pH-responsive nanoparticles had a faster cargo release than non-pH-responsive nanoparticles. Fluorescence showed the presence of the nanoparticles in the knee even after 35 days of administration. The group concluded that a combined therapy of pH-responsive and non-pH-responsive nanoparticles would have a synergistic effect for optimal therapeutic conditions. Therefore, pH-responsive nanoparticles would have a burst release and non-pH-responsive nanoparticles would have a gradual, steady release of the drug.
Zerrillo et al. conducted another study where they tested HA-grafted PLGA nanoparticles for OA therapy [77]. Conventional HA therapy has a rapid clearance and short retention time. Grafting is used as a method to overcome these issues. In this case, a 20 kDa HA is used to synthesize a PLGA-HA copolymer and prepare PLGA-HA nanoparticles. The nanoparticles showed a size of 200 nm. Near-infrared dye tests showed that PLGA-HA nanoparticles had a 20% lower release rate after 10 days. There was a two-fold increase in the in vitro binding studies for PLGA-HA nanoparticles. Intra-articular injection was used for in vivo tests. After 48 h, PLGA-HA nanoparticles were noted to have penetrated the cartilage, unlike PLGA nanoparticles without HA.
Histochemical immunostaining has reported OA synovium to have a higher number of CD44 receptors than normal. This leads to an increase in inflammation due to the active presence of pro-inflammatory cytokines. This has further led to the increased targeting of CD44 as a therapy for OA using HA, a well-known CD44 ligand [78][79][80][81].
3.3. Self-Assembling Nanosystems
The molecular arrangement of disorganised components into ordered structures as a response to external stimulus is known as self-assembly. It is a phenomenon often found in nature. Biological nanostructures come self-assembled to form a DNA double-helix, cell membranes, peptide chains, etc. [82][83][84].
Vafaei et al. tested a budesonide (BDS)-loaded self-assembled HA nanosystem as a therapeutic agent for inflamed intestinal mucosa caused by inflammatory bowel disease (IBD) [85]. The self-assembling effect was enhanced by amphiphilic chemical modifications to the HA backbone. The 10 and 25 kDa HAs were tested and the thin-film hydration method was used to load BDS. The human colon carcinoma cell line was used for in vitro tests. The increased degree of chemical modification led to a decrease in the size of nanoparticles. The size for 10 kDa HA decreased by 97 nm and, for 25 kDa HA, the size decreased by 61 nm.
Mota et al. synthesized a PLGA-loaded HA hybrid systems for viscosupplementation in OA [86]. The group used 1500–1800 kDa HA, 45–75 kDa PLGA and a modified spontaneous emulsification/solvent diffusion method for synthesis. Oleic acid was also used to propagate long-term controlled drug release and provide stability to the nanosystem. DLS was used to check the size of HA-loaded PLGA particles (373 nm) and oleic-acid-modified particles (4561 nm). The increase in the size of particles with oleic acid was attributed to particle agglomeration. Since DLS cannot differentiate between particle agglomeration and a large size, atomic force microscopy (AFM) was used to check the size of oleic-acid-modified particles, which showed a particle size of 409 nm. The use of the hybrid system for HA administration increased the drug release rate to up to 8 h, as compared to the instant dissolution of free HA. The in vivo anti-inflammatory effect was tested for free HA solution (76.9%) and HA-PLGA particles (82.6%).
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087286
References
- Pinho-Ribeiro, F.A.; Verri, W.A., Jr.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19.
- Kashem, S.W.; Riedl, M.S.; Yao, C.; Honda, C.N.; Vulchanova, L.; Kaplan, D.H. Nociceptive Sensory Fibers Drive Interleukin-23 Production from CD301b+ Dermal Dendritic Cells and Drive Protective Cutaneous Immunity. Immunity 2015, 43, 515–526.
- Calil, I.L.; Zarpelon, A.C.; Guerrero, A.T.G.; Alves-Filho, J.C.; Ferreira, S.H.; Cunha, F.Q.; Cunha, T.M.; Verri, W.A., Jr. Lipopolysaccharide Induces Inflammatory Hyperalgesia Triggering a TLR4/MyD88-Dependent Cytokine Cascade in the Mice Paw. PLoS ONE 2014, 9, e90013.
- Gabanyi, I.; Muller, P.; Feighery, L.; Oliveira, T.; Costa-Pinto, F.; Mucida, D. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391.
- Kurashige, C.; Hosono, K.; Matsuda, H.; Tsujikawa, K.; Okamoto, H.; Majima, M. Roles of receptor activity-modifying protein 1 in angiogenesis and lymphangiogenesis during skin wound healing in mice. FASEB J. 2013, 28, 1237–1247.
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Fukazawa, Y.; Tohya, K.; Kimura, M.; Kishioka, S. Epigenetic Augmentation of the Macrophage Inflammatory Protein 2/C-X-C Chemokine Receptor Type 2 Axis through Histone H3 Acetylation in Injured Peripheral Nerves Elicits Neuropathic Pain. J. Pharmacol. Exp. Ther. 2011, 340, 577–587.
- Kobayashi, Y.; Kiguchi, N.; Fukazawa, Y.; Saika, F.; Maeda, T.; Kishioka, S. Macrophage-T Cell Interactions Mediate Neuropathic Pain through the Glucocorticoid-induced Tumor Necrosis Factor Ligand System. J. Biol. Chem. 2015, 290, 12603–12613.
- Old, E.A.; Nadkarni, S.; Grist, J.; Gentry, C.; Bevan, S.J.; Kim, K.-W.; Mogg, A.J.; Perretti, M.; Malcangio, M. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J. Clin. Investig. 2014, 124, 2023–2036.
- Chen, O.; Donnelly, C.R.; Ji, R.-R. Regulation of pain by neuro-immune interactions between macrophages and nociceptor sensory neurons. Curr. Opin. Neurobiol. 2020, 62, 17–25.
- Shepherd, A.J.; Mickle, A.D.; Golden, J.P.; Mack, M.R.; Halabi, C.M.; de Kloet, A.D.; Samineni, V.K.; Kim, B.S.; Krause, E.G.; Gereau, R.W.; et al. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc. Natl. Acad. Sci. USA 2018, 115, E8057–E8066.
- Shutov, L.P.; Warwick, C.A.; Shi, X.; Gnanasekaran, A.; Shepherd, A.J.; Mohapatra, D.P.; Woodruff, T.M.; Clark, J.D.; Usachev, Y.M. The complement system component C5a produces thermal hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. J. Neurosci. 2016, 36, 5055–5070.
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80.
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256.
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276.
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 421–449.
- Liu, M.; Huang, Q.; Zhu, Y.; Chen, L.; Li, Y.; Gong, Z.; Ai, K. Harnessing reactive oxygen/nitrogen species and inflammation: Nanodrugs for liver injury. Mater. Today Bio 2022, 13, 100215.
- Sun, Q.; Ma, H.; Zhang, J.; You, B.; Gong, X.; Zhou, X.; Chen, J.; Zhang, G.; Huang, J.; Huang, Q.; et al. A Self-Sustaining Antioxidant Strategy for Effective Treatment of Myocardial Infarction. Adv. Sci. 2022, 10, 2204999.
- Antonelli, M.; Kushner, I. It’s time to redefine inflammation. FASEB J. 2017, 31, 1787–1791.
- Park, K.H.; Zaichenko, L.; Peter, P.; Davis, C.R.; Crowell, J.A.; Mantzoros, C.S. Diet quality is associated with circulating C-reactive protein but not irisin levels in humans. Metabolism 2013, 63, 233–241.
- Wu, W.-T.; Tsai, S.-S.; Shih, T.-S.; Lin, M.-H.; Chou, T.-C.; Ting, H.; Wu, T.-N.; Liou, S.-H. The impact of obstructive sleep apnea on high-sensitivity C-reactive protein in subjects with or without metabolic syndrome. Sleep Breath 2015, 19, 1449–1457.
- Heffner, K.L.; Waring, M.E.; Roberts, M.B.; Eaton, C.B.; Gramling, R. Social isolation, C-reactive protein, and coronary heart disease mortality among community-dwelling adults. Soc. Sci. Med. 2011, 72, 1482–1488.
- Cowman, M.K.; Lee, H.G.; Schwertfeger, K.L.; McCarthy, J.B.; Turley, E.A. The content and size of hyaluronan in biological fluids and tissues. Front. Immunol. 2015, 6, 261.
- Rayahin, J.E.; Buhrman, J.S.; Zhang, Y.; Koh, T.J.; Gemeinhart, R.A. High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng. 2015, 1, 481–493.
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.-H. The effects of the molecular weights of hyaluronic acid on the immune responses. Biomater. Res. 2021, 25, 27.
- Scaturro, D.; Vitagliani, F.; Terrana, P.; Tomasello, S.; Falco, V.; Cuntrera, D.; Spoto, I.; Midiri, M.; Mauro, G.L. Hybrid Hyaluronic Acid versus High Molecular Weight Hyaluronic Acid for the Treatment of Hip Osteoarthritis in Overweight/Obese Patients. J. Funct. Morphol. Kinesiol. 2022, 7, 20.
- Sharath, S.S.; Ramu, J.; Nair, S.V.; Iyer, S.; Mony, U.; Rangasamy, J. Human Adipose Tissue Derivatives as a Potent Native Biomaterial for Tissue Regenerative Therapies. Tissue Eng. Regen. Med. 2020, 17, 123–140.
- Yoon, J.-Y.; Kim, D.-W.; Ahn, J.-H.; Choi, E.-J.; Kim, Y.H.; Jeun, M.; Kim, E.-J. Propofol Suppresses LPS-Induced Inflammation in Amnion Cells via Inhibition of NF-κB Activation. Tissue Eng. Regen. Med. 2019, 16, 301–309.
- Erickson, M.; Stern, R. Chain gangs: New aspects of hyaluronan metabolism. Biochem. Res. Int. 2011, 2012, 893947.
- Bala, E.; Hazarika, R.; Singh, P.; Yasir, M.; Shrivastava, R. A biological overview of Hyaluronidase: A venom enzyme and its inhibition with plants materials. Mater. Today Proc. 2018, 5, 6406–6412.
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (CD44, RHAMM) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201.
- Chernos, M.; Grecov, D.; Kwok, E.; Bebe, S.; Babsola, O.; Anastassiades, T. Rheological study of hyaluronic acid derivatives. Biomed. Eng. Lett. 2017, 7, 17–24.
- Dhapte, V.; Pokharkar, V. Nanosystems for drug delivery: Design, engineering, and applications. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–345.
- Baeva, L.F.; Lyle, D.B.; Rios, M.; Langone, J.J.; Lightfoote, M.M. Different molecular weight hyaluronic acid effects on human macrophage interleukin 1β production. J. Biomed. Mater. Res. Part A 2014, 102, 305–314.
- Agas, D.; Laus, F.; Lacava, G.; Marchegiani, A.; Deng, S.; Magnoni, F.; Silva, G.G.; Di Martino, P.; Sabbieti, M.G.; Censi, R. Thermosensitive hybrid hyaluronan/p(HPMAm-lac)-PEG hydrogels enhance cartilage regeneration in a mouse model of osteoarthritis. J. Cell. Physiol. 2019, 234, 20013–20027.
- Isa, I.L.M.; Srivastava, A.; Tiernan, D.; Owens, P.; Rooney, P.; Dockery, P.; Pandit, A. Hyaluronic Acid Based Hydrogels Attenuate Inflammatory Receptors and Neurotrophins in Interleukin-1β Induced Inflammation Model of Nucleus Pulposus Cells. Biomacromolecules 2015, 16, 1714–1725.
- Chistyakov, D.V.; Astakhova, A.A.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. High and Low Molecular Weight Hyaluronic Acid Differentially Influences Oxylipins Synthesis in Course of Neuroinflammation. Int. J. Mol. Sci. 2019, 20, 3894.
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943.
- Mayumi, K.; Liu, C.; Yasuda, Y.; Ito, K. Softness, Elasticity, and Toughness of Polymer Networks with Slide-Ring Cross-Links. Gels 2021, 7, 91.
- Qian, Z.-Y.; Fu, S.-Z.; Feng, S.-S. Nanohydrogels as a prospective member of the nanomedicine family. Nanomedicine 2013, 8, 161–164.
- Suhail, M.; Rosenholm, J.M.; Minhas, M.U.; Badshah, S.F.; Naeem, A.; Khan, K.U.; Fahad, M. Nanogels as drug-delivery systems: A comprehensive overview. Ther. Deliv. 2019, 10, 697–717.
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126.
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533.
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded With Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell. Physiol. 2017, 232, 2063–2074.
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564.
- Storozhylova, N.; Crecente-Campo, J.; Cabaleiro, D.; Lugo, L.; Dussouy, C.; Simões, S.; Monteiro, M.; Grandjean, C.; Alonso, M.J. An In Situ Hyaluronic Acid-Fibrin Hydrogel Containing Drug-Loaded Nanocapsules for Intra-Articular Treatment of Inflammatory Joint Diseases. Regen. Eng. Transl. Med. 2020, 6, 201–216.
- Han, T.; Das, D.B. Potential of combined ultrasound and microneedles for enhanced transdermal drug permeation: A review. Eur. J. Pharm. Biopharm. 2015, 89, 312–328.
- Schoellhammer, C.M.; Blankschtein, D.; Langer, R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014, 11, 393–407.
- Gupta, S.; Bansal, R.; Gupta, S.; Jindal, N.; Jindal, A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol. Online J. 2013, 4, 267–272.
- Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010.
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521.
- Carbinatto, F.M.; de Castro, A.D.; Evangelista, R.C.; Cury, B.S. Insights into the swelling process and drug release mechanisms from cross-linked pectin/high amylose starch matrices. Asian J. Pharm. Sci. 2014, 9, 27–34.
- Ko, S.W.; Lee, J.Y.; Lee, J.; Son, B.C.; Jang, S.R.; Aguilar, L.E.; Oh, Y.M.; Park, C.H.; Kim, C.S. Analysis of Drug Release Behavior Utilizing the Swelling Characteristics of Cellulosic Nanofibers. Polymers 2019, 11, 1376.
- Hezaveh, H.; Muhamad, I.I.; Noshadi, I.; Fen, L.S.; Ngadi, N. Swelling behaviour and controlled drug release from cross-linked -carrageenan/NaCMC hydrogel by diffusion mechanism. J. Microencapsul. 2012, 29, 368–379.
- Wei, S.; Xie, J.; Luo, Y.; Ma, Y.; Tang, S.; Yue, P.; Yang, M. Hyaluronic acid based nanocrystals hydrogels for enhanced topical delivery of drug: A case study. Carbohydr. Polym. 2018, 202, 64–71.
- Fan, M.; Ma, Y.; Zhang, Z.; Mao, J.; Tan, H.; Hu, X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater. Sci. Eng. C 2015, 56, 311–317.
- Tan, H.; Fan, M.; Ma, Y.; Qiu, J.; Li, X.; Yan, J. Injectable Gel Scaffold Based on Biopolymer Microspheres via an Enzymatic Reaction. Adv. Healthc. Mater. 2014, 3, 1769–1775.
- Liu, S.; Zhang, Q.; Yu, J.; Shao, N.; Lu, H.; Guo, J.; Qiu, X.; Zhou, D.; Huang, Y. Absorbable Thioether Grafted Hyaluronic Acid Nanofibrous Hydrogel for Synergistic Modulation of Inflammation Microenvironment to Accelerate Chronic Diabetic Wound Healing. Adv. Healthc. Mater. 2020, 9, e2000198.
- Pleguezuelos-Villa, M.; Nacher, A.; Hernández, M.J.; Buso, M.O.V.; Sauri, A.R.; Díez-Sales, O. Mangiferin nanoemulsions in treatment of inflammatory disorders and skin regeneration. Int. J. Pharm. 2019, 564, 299–307.
- Manca, M.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109.
- Yang, Y.; Qiu, D.; Liu, Y.; Chao, L. Topical anesthetic analgesic therapy using the combination of ropivacaine and dexmedetomidine: Hyaluronic acid modified long-acting nanostructured lipid carriers containing a skin penetration enhancer. Drug Des. Dev. Ther. 2019, 13, 3307–3319.
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165.
- Yue, Y.; Zhao, D.; Yin, Q. Hyaluronic acid modified nanostructured lipid carriers for transdermal bupivacaine delivery: In vitro and in vivo anesthesia evaluation. Biomed. Pharmacother. 2018, 98, 813–820.
- Iannitti, T.; Morales-Medina, J.C.; Merighi, A.; Boarino, V.; Laurino, C.; Vadalà, M.; Palmieri, B. A hyaluronic acid- and chondroitin sulfate-based medical device improves gastritis pain, discomfort, and endoscopic features. Drug Deliv. Transl. Res. 2018, 8, 994–999.
- Farajzadeh, R.; Zarghami, N.; Serati-Nouri, H.; Momeni-Javid, Z.; Farajzadeh, T.; Jalilzadeh-Tabrizi, S.; Sadeghi-Soureh, S.; Naseri, N.; Pilehvar-Soltanahmadi, Y. Macrophage repolarization using CD44-targeting hyaluronic acid–polylactide nanoparticles containing curcumin. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2013–2021.
- Zhang, L.; Wang, J.; Chi, H.; Wang, S. Local anesthetic lidocaine delivery system: Chitosan and hyaluronic acid-modified layer-by-layer lipid nanoparticles. Drug Deliv. 2016, 23, 3529–3537.
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699.
- Tran, T.-H.; Rastogi, R.; Shelke, J.; Amiji, M.M. Modulation of Macrophage Functional Polarity towards Anti-Inflammatory Phenotype with Plasmid DNA Delivery in CD44 Targeting Hyaluronic Acid Nanoparticles. Sci. Rep. 2015, 5, 16632.
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502.
- Kosovrasti, V.Y.; Nechev, L.V.; Amiji, M.M. Peritoneal Macrophage-Specific TNF-α Gene Silencing in LPS-Induced Acute Inflammation Model Using CD44 Targeting Hyaluronic Acid Nanoparticles. Mol. Pharm. 2016, 13, 3404–3416.
- Xie, J.; Ji, Y.; Xue, W.; Ma, D.; Hu, Y. Hyaluronic acid-containing ethosomes as a potential carrier for transdermal drug delivery. Colloids Surf. B Biointerfaces 2018, 172, 323–329.
- Hussain, A.; Haque, W.; Singh, S.K.; Ahmed, F.J. Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using Design Expert: Part II. Drug Deliv. 2015, 23, 1242–1253.
- Li, Y.; Xu, F.; Li, X.; Chen, S.-Y.; Huang, L.-Y.; Bian, Y.-Y.; Wang, J.; Shu, Y.-T.; Yan, G.-J.; Dong, J.; et al. Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int. J. Pharm. 2020, 592, 119936.
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal applications of ethosomes—A detailed review. J. Liposome Res. 2018, 29, 103–113.
- Gao, Y.; Cheng, X.; Wang, Z.; Wang, J.; Gao, T.; Li, P.; Kong, M.; Chen, X. Transdermal delivery of 10,11-methylenedioxycamptothecin by hyaluronic acid based nanoemulsion for inhibition of keloid fibroblast. Carbohydr. Polym. 2014, 112, 376–386.
- Brun, P.; Zavan, B.; Vindigni, V.; Schiavinato, A.; Pozzuoli, A.; Iacobellis, C.; Abatangelo, G. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500-730 kDa hyaluronan amide derivative. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2073–2081.
- Zerrillo, L.; Que, I.; Vepris, O.; Morgado, L.; Chan, A.; Bierau, K.; Li, Y.; Galli, F.; Bos, E.; Censi, R.; et al. pH-responsive poly(lactide-co-glycolide) nanoparticles containing near-infrared dye for visualization and hyaluronic acid for treatment of osteoarthritis. J. Control. Release 2019, 309, 265–276.
- Zerrillo, L.; Gigliobianco, M.R.; D’Atri, D.; Garcia, J.P.; Baldazzi, F.; Ridwan, Y.; Fuentes, G.; Chan, A.; Creemers, L.B.; Censi, R.; et al. PLGA Nanoparticles Grafted with Hyaluronic Acid to Improve Site-Specificity and Drug Dose Delivery in Osteoarthritis Nanotherapy. Nanomaterials 2022, 12, 2248.
- Badghaish, M.M.O.; Qorban, G.N.M.; Albaqami, A.S.; Nemer, A.A.; Alali, A.J.; Al Yaqoub, R.F.H.; Alshamrani, H.A.; Badahman, O.H.; Ansaif, R.A.; Alasmari, M.A.; et al. Rheumatoid Arthritis, Pathophysiology and Management. Egypt. J. Hosp. Med. 2018, 70, 1898–1903.
- Gorantla, S.; Gorantla, G.; Saha, R.N.; Singhvi, G. CD44 receptor-targeted novel drug delivery strategies for rheumatoid arthritis therapy. Expert Opin. Drug Deliv. 2021, 18, 1553–1557.
- Altman, R.; Manjoo, A.; Fierlinger, A.; Niazi, F.; Nicholls, M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 321.
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236.
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 127.
- Mendes, A.C.; Baran, E.T.; Reis, R.L.; Azevedo, H.S. Self-assembly in nature: Using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 582–612.
- Stoffelen, C.; Huskens, J. Soft Supramolecular Nanoparticles by Noncovalent and Host–Guest Interactions. Small 2015, 12, 96–119.
- Vafaei, S.Y.; Esmaeili, M.; Amini, M.; Atyabi, F.; Ostad, S.N.; Dinarvand, R. Self assembled hyaluronic acid nanoparticles as a potential carrier for targeting the inflamed intestinal mucosa. Carbohydr. Polym. 2016, 144, 371–381.
- Mota, A.H.; Direito, R.; Carrasco, M.P.; Rijo, P.; Ascensão, L.; Viana, A.S.; Rocha, J.; Eduardo-Figueira, M.; Rodrigues, M.J.; Custódio, L.; et al. Combination of hyaluronic acid and PLGA particles as hybrid systems for viscosupplementation in osteoarthritis. Int. J. Pharm. 2019, 559, 13–22.
This entry is offline, you can click here to edit this entry!
