The tryptophan and kynurenine pathway is well-known to play an important role in nervous, endocrine, and immune systems, as well as in the development of inflammatory diseases. It has been documented that some kynurenine metabolites are considered to have anti-oxidative, anti-inflammatory, and/or neuroprotective properties. Importantly, many of these kynurenine metabolites may possess immune-regulatory properties that could alleviate the inflammation response. The abnormal activation of the tryptophan and kynurenine pathway might be involved in the pathophysiological process of various immune-related diseases, such as inflammatory bowel disease, cardiovascular disease, osteoporosis, and/or polycystic ovary syndrome.
1. Introduction
Among the 20 amino acids that supply protein formation, tryptophan is one of the essential mammalian amino acids, and the kynurenine pathway is the major metabolic pathway of the tryptophan metabolism, which is known to play an important role in the nervous, endocrine, and immune systems [
1]. The tryptophan metabolism is also closely related to inflammation and inflammatory diseases such as infection, coronary heart disease, autoimmune syndrome, cancer, and neurodegenerative disorders [
2]. Similarly, immune activation is intricately linked with the dysregulation of the tryptophan metabolism on the way to oxidative breakdown along the kynurenine axis [
2]. In addition, the amino acids in the tryptophan–kynurenine pathway have been shown to be associated with the risks of atherosclerotic cardiovascular events, which may predict acute coronary events in older individuals without previous coronary heart disease. [
3]. Furthermore, accumulating evidence has also suggested a role of the kynurenine pathway in various diseases and disorders, including Alzheimer’s Disease, depression, schizophrenia, amyotrophic lateral sclerosis, Huntington’s Disease, and/or cancers [
4]. The tryptophan–kynurenine pathway is the most important course for the tryptophan catabolism, accounting for around 95% of dietary tryptophan degradation [
5]. As a consequence of the kynurenine pathway’s metabolic integration, inflammation could contribute to the accumulation of kynurenine in the host brain, which may be associated with depression and/or schizophrenia [
5].
Dependent on the nutritional availability of the essential amino acid tryptophan, bacteria of the gut microbiota could influence the host’s serotonin biosynthesis in enterochromaffin cells. Therefore, certain members of the gut microbiota might be capable of delivering serotonin to their host [
6]. An increase in serotonin levels, induced by spore-forming bacteria in the gut, might contribute to the progression of inflammation, with an impact on neutrophil function [
7]. An additional tryptophan-dependent pathway that is modulated by the gut microbiota is the indole pathway, which also impacts mucosal Th17 cells’ immunity in the gut via the aryl hydrocarbon receptor (AHR) [
8,
9]. In addition, the tryptophan–kynurenine pathway might underlie the regulation performed by gut microbiota-regulated interferons [
10]. Meaningfully, a protective role of dietary tryptophan has been demonstrated in mouse models [
11].
2. Tryptophan and Kynurenine Metabolic Pathway
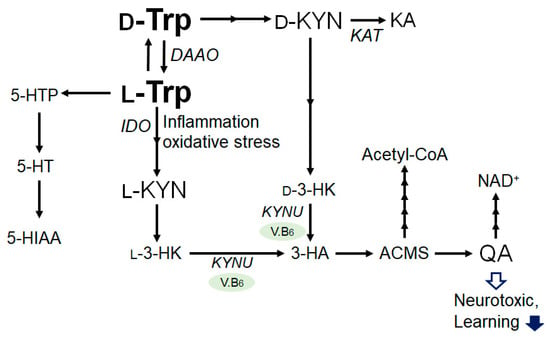
The catabolism of tryptophan has two major enzymatic pathways. The first pathway is the serotonin pathway, which is mediated by tryptophan hydroxylase and results in the generation of serotonin or 5-hydroxytryptamin (5-HT), a precursor of melatonin [
12]. In the other pathway, tryptophan is catabolized to kynurenine, the neuroprotective kynurenic acid, and the neurotoxic quinolinic acid (QA) [
13]. Interestingly, D-tryptophan can also be enzymatically converted to L-tryptophan by D-amino acid oxidase and aminotransferase (
Figure 1). Tryptophan is mainly catabolised through the kynurenine pathway [
14], which is controlled at its first step and catalyzed by the rate-determining enzymes, tryptophan 2,3-dioxygenase in the liver and indoleamine 2,3-dioxygenase in the extrahepatic tissues [
15]. The tryptophan and kynurenine pathway accounts for the majority of ingested tryptophan [
12,
16]. Kynurenine could be further metabolized into several other downstream products, such as 3-hydroxykynurenine, xanthurenic acid, anthranilic acid, 3-hydroxyanthranilic acid, and picolinic acid, which are collectively referred to as kynurenines. Kynurenine is converted into anthranilic acid by kynureninase, and to 3-hydroxykynurenine by kynurenine monooxygenase. Then, 3-hydroxykynurenine is metabolized into 3-hydroxyanthranilic acid by kynureninase. Lastly, quinolinic acid is generated, and thereafter, so is nicotinamide adenine dinucleotide (NAD), which plays an essential role in energy metabolism. The pathway involving the indoleamine-2,3-dioxygenase has been assumed to play a major role in the development of major depression. In addition, the tryptophan 2,3-dioxygenase activity is inhibited by the glucose intake in liver, probably involving the increased production of the feedback allosteric inhibitor, NADPH [
17]. This may explain the associations between the elevated concentrations of kynurenine metabolites with the impaired glucose tolerance observed in individuals that are overweight or have obesity [
18].
Figure 1. Illustration of the general L-Tryptophan and L-Kynurenine or D-Tryptophan and D-Kynurenine metabolic pathway in bacteria and/or in mammals. Some enzymatic degradation has also been shown. The arrowhead means stimulation and/or augmentation. As a footnote, some critical events have been omitted for simplicity. D-Trp: D-Tryptophan; L-Trp: L-Tryptophan; D-KYN: D-Kynurenine; L-KYN: L-Kynurenine; KA: Kynurenic acid; l-3-HK: l-3-Hydroxykynurenine, d-3-HK: d-3-Hydroxykynurenine; 3-HA: 3-Hydroxyanthranilic acid; ACMSD: α-amino-β-carboxymuconate-ε-semialdehyde; QA: Quinolinic acid; NAD: Nicotinamide adenine dinucleotide; 5-HTTP: 5-Hydroxytryptophan; 5-HTP: 5-Hydroxytryptophan; 5-HT: 5-hydroxytryptamine (Serotonin); 5-HIAA: 5-Hydroxyindoleacetic acid; V.B6: vitamin B6; DAAO: d-amino acid oxidase; IDO: Indoleamine 2,3-dioxygenase; KAT: kynurenine aminotransferase; and KYNU: kynureninase.
Some kynurenines are considered to have anti-oxidative, anti-inflammatory, and neuroprotective properties, while others have pro-oxidative, pro-inflammatory, and neurotoxic properties, and others have somewhat less well-characterized properties [
20]. The tryptophan–kynurenine pathway is recognized to be involved in quality of life (QOL) after cancer [
5,
21,
22], which may play a crucial role in inflammation [
23]. In addition, the kynurenine pathway determines the overall neuronal excitability and plasticity by modulating the glutamate receptors and G protein-coupled receptor 35 (GPR35) activity across the central nervous system (CNS), and regulating the general features of immune cell status, surveillance, and tolerance, which often involves the AHR. The ratio of kynurenine-to-tryptophan may be a recognized marker of cellular immune activation [
24].
3. Connection between Kynurenine Pathway and Immunity
Inflammatory cytokines could activate indoleamine 2,3-dioxygenase. For example, interferon gamma can activate the enzyme of indoleamine 2,3-dioxygenase, thereby shifting the Trp metabolism to Kyn production [
26]. Cytokines and kynurenines are closely associated with mediating the communication between the brain and the immune system, which could regulate the neuron and/or glial activity in the central nervous system (CNS), as well as the function of the immune system within a combined network. This concept would broaden the scope for the development of new treatments for disorders that involve immune systems with harmless and/or more active agents [
27]. During inflammation, pro-inflammatory cytokines, mainly tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), may increase the catabolism of tryptophan by encouraging the expression of indoleamine 2,3-dioxygenase [
28]. Increasing evidence may verify inflammation as key for the entry of tryptophan into the kynurenine pathway, and, in return, this pathway might metabolite as a crucial modulator of inflammatory responses. The kynurenine pathway might also be activated to generate sufficient amounts of NAD+ energy, as activated immune cells may need a great deal of energy to fight against pathogens [
29], which could protect the host against the oxidative stresses that are linked to various inflammations. Significantly, a lot of the kynurenine metabolites may have the potential for immune regulation, which could alleviate the inflammation. For example, kynurenic acid could reduce pro-inflammatory cytokines [
30].
4. Several Immune-Related Diseases Associated with the Tryptophan and Kynurenine Pathway
4.1. Major Depressive and Bipolar Disorders
The metabolism of tryptophan, which is the precursor of neurotransmitters, has been deliberated to be one of the important biological pathways for depression [
39]. Possibly based on the relationship of the tryptophan metabolism with mental disorders, inflammatory diseases are frequently comorbid with major depression. In general, the dysregulation of tryptophan metabolites, such as serotonin, kynurenine, quinolinic acid, and kynerunic acid, might also be interrelated to depressive disorders.
4.2. Cardiovascular Disease
Cardiovascular diseases, including myocardial infarction and/or stroke, may constitute a leading cause of death, globally. The pathophysiology of cardiovascular diseases is linked to vascular inflammation [
43], which may be linked to hypertension. Aryl hydrocarbon receptor (AHR)-signaling is well recognized to contribute to those cardiovascular pathologies by inducing the expression of pro-inflammatory interleukin IL-1β, IL-8, and TNF-α, with the following foam cells [
44]. Therefore, the tryptophan metabolites may also have an influence on vascular inflammation [
45].
4.3. Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease
Kidney diseases, including acute kidney injury and/or chronic kidney disease, may represent a worldwide health issue [
48,
49,
50]. The kynurenine pathway might also be activated in acute kidney injury [
51] and the kidney tissues of mice with ischemia/reperfusion-induced acute kidney injury [
52]. Consistently, mice with acute kidney injury arising from drug-induced toxicity might have a higher amount of kynurenine [
53].
4.4. Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), which predominantly encompasses ulcerative colitis (UC) and Crohn’s disease, is a collective chronic intestinal inflammatory disease. Clinical studies have shown that the tryptophan metabolism might be associated with the severity of IBD [
55]. A tryptophan deficiency might contribute to the development of IBD and/or aggravate a disease’s activity/severity [
55]. In addition, a strong correlation between kynurenine levels and IBD has been demonstrated, which would provide new insights into IBD pathogenesis [
56]. The modeling of the tryptophan metabolite fluxes in IBD has indicated that changes in gene expression shifted the intestinal tryptophan metabolism from the synthesis of serotonin towards the kynurenine pathway [
57].
4.5. Osteoporosis
Osteoporosis is also associated with the abnormalities of the kynurenine pathway [
59]. Osteoporosis is a highly prevalent disease which is characterized by a low bone mass and represents the most common cause for bone fractures in the elderly [
60]. A factor for the pathogenesis in osteoporosis may be the imbalanced activities of osteoblasts and osteoclasts [
61]. Recent studies of T cells and osteoporosis have suggested the involvement of some T-cell subsets, regulatory T (Treg) cell and T helper 17 (Th17) cell subsets [
62].
4.6. Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is an intricate metabolic disorder that is regarded in women of reproductive age. Low-grade inflammatory situations, such as obesity and a compromised glucose tolerance, may act as collective metabolic disturbances in females with PCOS. Furthermore, the elevations of inflammatory cytokines, such as chemokines and/or interleukins, may worsen this metabolic disturbance in inflammatory disease patients with PCOS [
67]. The pathology of PCOS may be a multifactorial disorder in the ovarian folliculogenesis, compromised gonadotropin levels, a genetic predisposition, and/or a gut microbiota imbalance. Inflammation appears to be the shared property between PCOS and the kynurenine pathway [
68].
5. Kynurenine Metabolites Involved in Brain Memory System and Immunity
Tryptophan may be altered into several bioactive molecules, such as serotonin in the brain. However, more than 90% of tryptophan might be altered into kynurenine, in a process recognized as the kynurenine pathway [
71]. Only a small amount of tryptophan may be metabolized into serotonin. In general, serotonin has been well-known as a monoamine neurotransmitter that could play an important role in several complex biological functions. In the brain, the biological roles of serotonin may include the functions of learning and/or memory [
71,
72]. In addition, it has been reported that kynurenine metabolites could also play roles in memory via the regulation of neurotransmitter systems. For instance, kynurenine and quinolinic acid (QA) have contrasting effects on NMDA receptor function [
73]. Quinolinic acid may contribute to the stimulation of the NMDA receptor by inhibiting glutamate re-uptake [
74]. Likewise, quinolinic acid has been revealed to activate the release of glutamate from neurons, preventing its re-uptake by astrocytes [
75].
In the brain, the tryptophan metabolite kynurenic acid functions as an endogenous antagonist of glycine co-agonist sites in the NMDA receptor at endogenous brain concentrations [
80]. The NMDA receptor is critical for the regulation of synaptic plasticity and/or several cognitive functions [
81]. In particular, NMDA receptors in the hippocampal CA3 are required for the artificial association of memory events that are stored in the CA3 cell ensembles [
82]. In addition, the NMDA-receptor-mediated structural plasticity of dendritic spines plays an important role in synaptic transmission in the brain during learning and memory formation [
83], which is an important role for the endogenous kynurenic acid in the control of GABAergic neurotransmission in the prefrontal cortex [
84].
Kynurenic acid is also a ligand of the GPR35 [
89] and is able to activate the AHR [
89]. The GPR35 is massively expressed in immune cells such as eosinophils, monocytes, natural killer-like T cells, and/or gastrointestinal cells, suggesting that the GPR35 might be physiologically important in these cells [
90]. In addition, the GPR35 is upregulated in activated neutrophils, which could promote their migration [
91]. It has been revealed that serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) might be a ligand of the GPR35 [
91]. Interestingly, tryptophan raised the levels of kynurenine, kynurenic acid, and 5-HIAA in a time-dependent manner [
92]. The AHR is a transcription factor that incorporates dietary, microbial, metabolic, and/or environmental signals to control intricate cellular performances. The postbiotics of the gut microbiota metabolism may be a significant source of nutritional AHR ligands [
93]. Short-chain fatty acids (SCFAs) are typical examples of postbiotics that may include acetate, butyrate, and propionate, which are derived from the fermentation of dietary fibers by gut microbiota that could activate the AHR [
94]. The AHR pathway may be involved in several immune processes which are dynamic for the host’s intestinal homeostasis, as well as for the optimal microbiome [
95].
Some of the distinctive kynurenine aminotransferases could catalyze the transamination of L-kynurenine (L-KYN) to kynurenic acid [
99]. Kynurenic acid can be formed from D-kynurenine (D-KYN) through an oxidative deamination by D-amino acid oxidase [
100], which may account, in part, for the kynurenic acid synthesis from d-kynurenine in the brain [
100]. Therefore, de novo kynurenic acid formation could involve different mechanisms. In particular, ROS should be considered as a possible alternative for the production of kynurenic acid from both L-KYN and D-KYN under physiological and/or pathological conditions [
101].
Microglia have the ability to mediate innate immune memory and can be reprogrammed by primary stimuli to enhance or inhibit their immune response to secondary stimuli. Inflammatory stimulation is an important factor for microglia to mediate innate immune memory. Single or repeated stimulation can induce the microglia to form different phenotypes. This microglia-mediated innate immune response is involved in the regulation of immune memory. The neuronal deletion of excitatory amino acid transporter 2 may lead to the dysregulation of the kynurenine pathway, and the astrocytic deficiency of excitatory amino acid transporter 2 may also result in the dysfunction of innate and adaptive immune pathways, which correlate with cognitive decline [
105].
This entry is adapted from the peer-reviewed paper 10.3390/ijms24065742