Dendrimers are three-dimensional nanostructures with a high degree of molecular homogeneity, adjustable size, multivalence, high surface functionality, and high aqueous solubility. Due to these important and attractive properties, dendrimers are already being used to deliver a number of drugs and are being explored as promising carriers for nucleic acid-based vaccines. Here summarizes the literature data on the biosafety of some dendrimers has been evaluated in several clinical trials.
- dendrimer
- drugs delivery
- safety and efficiency
- clinical trials
1. Introduction
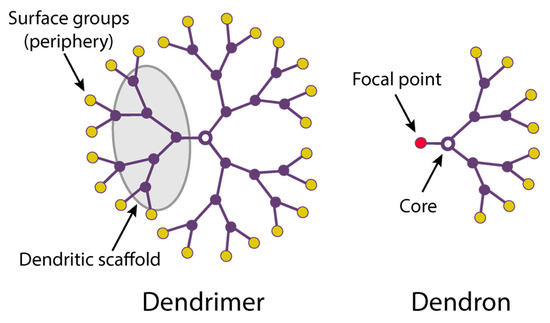
2. The Use of Dendrimers for Biomedical Applications
Distinct dendrimer-based constructions have been subjected to clinical trials; their safety and efficacy have been demonstrated in Phases 1–3, although some studies are still ongoing. Table 1 lists the dendrimer-based products involved in clinical trials, according to the clinicaltrials.gov database. These examples clearly highlight the growing employment of dendrimer-based drug delivery systems in the pharmaceutical industry.
Table 1. Dendrimer-based drugs in clinical trials.
| Drug | Description of the Drug | Study Title | Study Dates | Brief Study Description | Company | Clinicaltrials.gov Identifier | Ref. |
|---|---|---|---|---|---|---|---|
| SPL-7013 Gel (VivaGel™) | G4 poly(L-lysine) dendrimer bearing 32 sodium 1-(carboxymethoxy) naphthalene 3,6-disulfonate on the surface | SPL7013 gel—male tolerance study | August 2006–June 2007 | A phase 1, placebo-controlled study of the safety of a 3% w/w SPL7013 gel, administered to the penis of healthy male volunteers once daily for seven days | Starpharma Pty Ltd., Abbotsford, Australia | NCT00370357 | [61] |
| SPL-7013 Gel (VivaGel™) | “ | VivaGel™ in healthy young women | December 2006–November 2007 | A phase 1, expanded, randomized placebo-controlled trial of the safety and tolerability of a 3% w/w SPL7013 gel in healthy young women when administered twice daily for 14 days | Starpharma Pty Ltd., Abbotsford, Australia | NCT00331032 | [62] |
| SPL-7013 Gel (VivaGel™) | “ | Safety and acceptability of SPL7013 gel (VivaGel™) in sexually active women | July 2007–December 2009 | A phase 1 study of the safety and acceptability of a 3% w/w SPL7013 Gel applied vaginally in sexually active young women | Starpharma Pty Ltd., Abbotsford, Australia | NCT00442910 | [63] |
| SPL-7013 Gel (VivaGel™) | “ | Retention and duration of activity of SPL7013 (VivaGel®) after vaginal dosing | August 2008–March 2009 | Phase 1 and phase 2 assessments of local retention and duration of activity following vaginal application of a 3% VivaGel in healthy volunteers | Starpharma Pty Ltd., Abbotsford, Australia | NCT00740584 | [64] |
| SPL-7013 Gel (VivaGel™) | “ | Dose-ranging study of SPL7013 gel for treatment of bacterial vaginosis (BV) | August 2010–May 2011 | A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of the VivaGel administered vaginally in the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01201057 | [65] |
| SPL-7013 Gel (VivaGel™) | “ | Dose-ranging study of SPL7013 gel for the prevention of bacterial vaginosis (BV) | August 2011–December 2012 | A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study to determine the efficacy and safety of the SPL7013 gel administered vaginally to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01437722 | [66] |
| SPL-7013 Gel (VivaGel™) | “ | A phase 3 study of SPL7013 gel (VivaGel) for the treatment of bacterial vaginosis | April 2012–October 2012 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to assess the efficacy and safety of a 1% SPL7013 gel for the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01577537 | [67] |
| SPL-7013 Gel (VivaGel™) | “ | A phase 3 study of SPL7013 gel (VivaGel) for the treatment of bacterial vaginosis | March 2012–July 2012 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to assess the efficacy and safety of a 1% SPL7013 gel for the treatment of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT01577238 | [67] |
| SPL-7013 Gel (VivaGel™) | “ | Efficacy and safety study of SPL7013 gel to prevent the recurrence of bacterial vaginosis (BV) | October 2014–October 2016 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to determine the efficacy and safety of the SPL7013 gel to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT02236156 | [68] |
| SPL-7013 Gel (VivaGel™) | “ | Efficacy and safety study of SPL7013 gel to prevent the recurrence of bacterial vaginosis (BV) | October 2014–February 2017 | A phase 3, double-blind, multicenter, randomized, placebo-controlled study to determine the efficacy and safety of the SPL7013 gel to prevent the recurrence of bacterial vaginosis | Starpharma Pty Ltd., Abbotsford, Australia | NCT02237950 | [69] |
| AZD0466 | Astra Zeneca cancer drug AZD4320, chemically conjugated to a PEGylated poly-lysine dendrimer | A Study of AZD0466 in patients with advanced hematologic or solid tumors | December 2019–June 2021 | A phase 1, first-in-human study to determine the safety, tolerability, maximum tolerated dose (MTD), recommended Phase 2 dose (RP2D), and pharmacokinetics (PK) of AZD0466 in patients with solid tumors, lymphoma, and multiple myeloma at low, intermediate, or high risk for tumor lysis syndrome (TLS) with hematologic malignancies for whom no standard therapy exists | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cambridge |
NCT04214093 | [70] |
| AZD0466 | “ | A phase I/II study of AZD0466 as monotherapy or in combination with anticancer agents in advanced non-Hodgkin lymphoma | July 2022–November 2024 [Estimated] | A phase 1/2, modular, open-label, dose escalation and expansion, multicenter study of the safety, tolerability, PK, and preliminary efficacy of AZD0466 as a monotherapy, or in combination with other anticancer agents in patients with advanced NHL | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cambridge |
NCT05205161 | [71][72] |
| AZD0466 | “ | Study of AZD0466 monotherapy or in combination in patients with advanced hematological malignancies | June 2021–June 2024 [Estimated] | A phase 1/2, modular, open-label, multicenter study to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of AZD0466 as a monotherapy and drug-drug interaction potential between AZD0466 and the azole antifungal voriconazole in participants with advanced hematological malignancies | Starpharma, Abbotsford, Australia; AstraZeneca, UK, Cam-bridge |
NCT04865419 | [73] |
| ImDendrim | G5 polylysine dendrimer mixed with nitro-imidazole-methyl-1,2,3-triazol-methyl-di-(2-pycolyl) amine | Treatment of non-responding to conventional therapy inoperable liver cancers by in situ introduction of ImDendrim | March 2017–December 2017 | An open-label and unicenter study in patients with primary hepatocellular cancer or metastatic liver cancer without standard therapeutic options for treatment, including chemotherapy or surgery | National Institute of Allergy and Infectious Diseases (NIAID), North Bethesda, Maryland, USA | NCT03255343 | [74] |
| OP-101 | G4 PAMAM dendrimer N-acetyl-cysteine | A study to evaluate the safety, tolerability, and pharmacokinetics of OP-101 after intravenous administration in healthy volunteers | March 2018–July 2018 | A phase 1, open-label single ascending dose study to evaluate the safety, tolerability, and pharmacokinetics after intravenous administration in healthy volunteers | Orpheris, Inc. Redwood City, California, USA |
NCT03500627 | [75] |
| OP-101 | “ | A clinical study to measure the effect of OP-101 after being administered subcutaneous in healthy volunteers | March 2020–May 2020 | A phase 1, open-label single ascending dose study to evaluate the safety, tolerability, and pharmacokinetics after subcutaneous administration in healthy volunteers | Orpheris, Inc. Redwood City, California, USA |
NCT04321980 | [76] |
| OP-101 | “ | A study to evaluate OP-101 (dendrimer N-acetyl-cysteine) in severe coronavirus disease 2019 (COVID-19) patients (PRANA) | August 2020–August 2022 [Estimated] | A phase 2, two-stage, double-blind, placebo-controlled study to evaluate the safety, tolerability, pharmacokinetics, and efficacy in patients with severe COVID-19 | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT04458298 | [77] |
| D-4517.2 | Hydroxyl dendrimer, VEGFR tyrosine kinase inhibitor | A study to evaluate the safety, tolerability, and pharmacokinetics of D-4517.2 after subcutaneous administration in healthy participants | January 2022–August 2022 | A phase 1, open-label, single-ascending dose study of the safety, tolerability, and pharmacokinetics after subcutaneous administration in healthy volunteers | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT05105607 | [78] |
| D-4517.2 | “ | A study to evaluate the safety, tolerability, and pharmacokinetics of D-4517.2 after subcutaneous administration in subjects with neovascular (Wet) age-related macular degeneration (AMD), or subjects with diabetic macular edema (DME) (Tejas) | August 2022–June 2023 [Estimated] | A phase 2, two-stage study: open-label assessment of safety and pharmacodynamic response as well as a visual examiner-masked, randomized active, sham, and placebo controlled study evaluating the efficacy of D-4517.2 administered subcutaneously to subjects with neovascular age-related macular degeneration or subjects with diabetic macular edema | Ashvattha Therapeutics, Inc. Redwood City, California, USA | NCT05387837 | [79] |
| siCoV/KK46 | Anti-SARS-CoV-2 siRNA (targeting RNA-dependent RNA polymerase)/KK-46 (peptide dendrimer) complex | The siCoV/KK46 drug open-safety study | January 2021–March 2021 | A phase 1, open-label, dose-escalation study to assess the safety and tolerability of single and multiple doses in healthy volunteers (inhalation use) | National Research Center —Institute of Immunology FMBA, Saint Petersburg, Russia | NCT05208996 | [80] |
| MIR 19® | “ | Evaluation of safety and efficacy of a MIR 19 ® inhalation solution in patients with moderate COVID-19 | April 2021–September 2021 | A phase 2, multicenter controlled randomized study to assess the efficacy and safety of MIR 19® via 14 days of treatment of participants with symptomatic moderate COVID-19 | National Research Center—Institute of Immunology FMBA, Saint Petersburg, Russia | NCT05184127 | [81] |
The first dendrimer-based, anti-microbial drug approved for use in humans was Vivagel® (SPL7013) (Starpharma, Abbotsford, Australia), a poly-L-lysine dendrimer derivastive used as a topical microbicide for the prevention and treatment of bacterial vaginosis (BV). There have been at least 13 clinical studies of this product, with the first clinical trial completed in 2004. SPL7013 is a polyanionic dendrimer with a benzylhydramine lysine core and has 32 naphthalene disulfonate surface functionalities (Figure 2). This product also provides the prevention of genital herpes (HSV-2), HIV, and other sexually transmitted infections (STIs). Studies have shown that SPL7013 is generally well tolerated with minimal side effects [82][83][84][85][86][87][88][89][90][91]. SPL7013, in the form of a nasal spray called VIRALEZE, has also undergone a first growth phase as registered in the Australian and New Zealand Clinical Trials Registry [92].
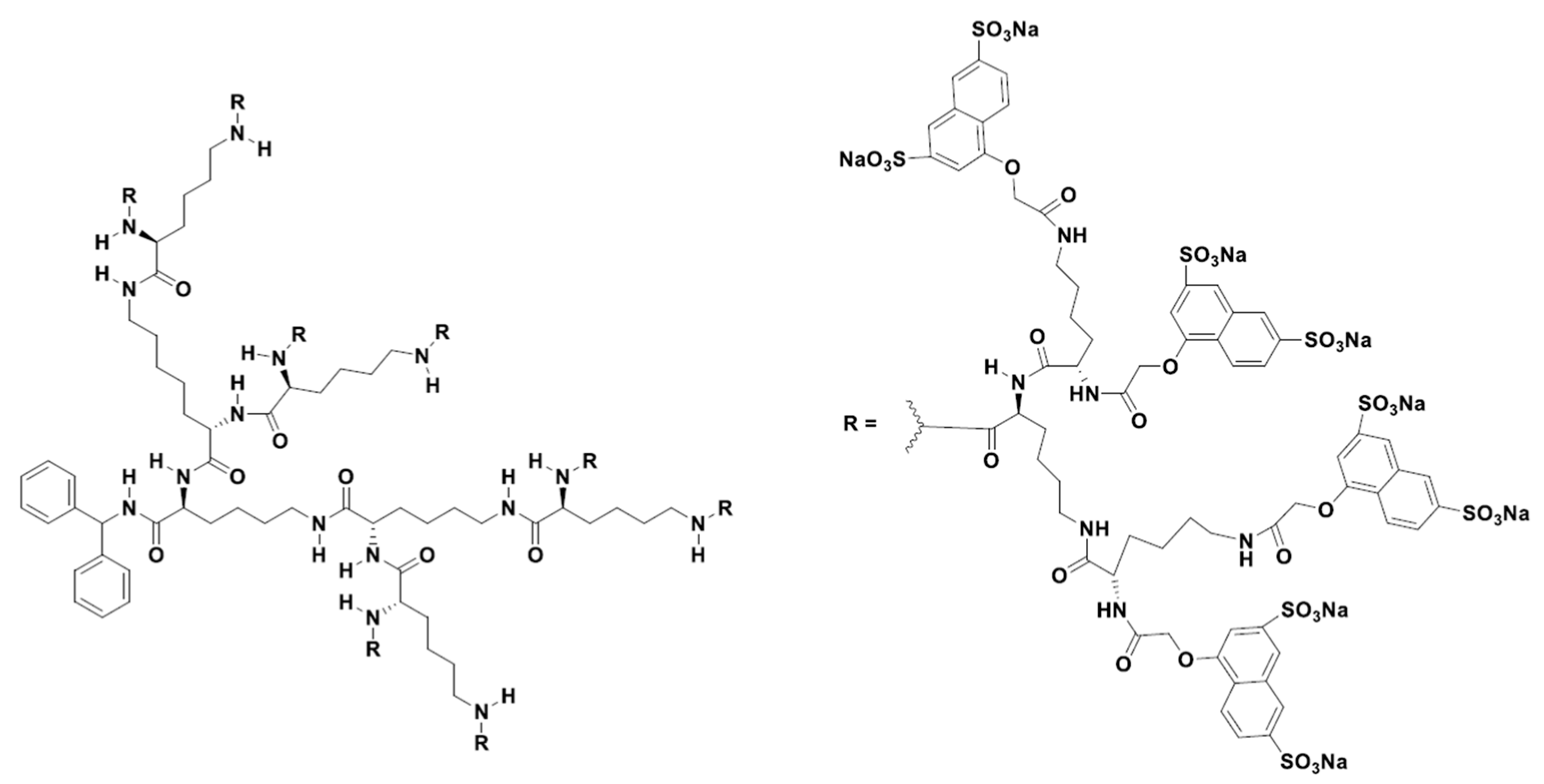
Figure 2. Two-dimensional chemical structure of VivaGel® [92].
Another example of a poly-L-lysine dendrimer developed by Starpharma and AstraZeneca is AZD0466. This is a fifth generation poly-L-lysine dendrimer, a highly optimized molecule containing the anti-cancer drug AZD4320, and a PEGylated poly-L-lysine dendrimer. AZD0466 belongs to a new class of oncology drugs that provide efficient delivery of a dual Bcl-2/xL inhibitor with an optimized release profile that is designed to reduce the potential for toxicity associated with dual Bcl-2/xL inhibition [93][94][95][96]. The AZD0466 construct is currently undergoing simultaneous phase1/phase 2 clinical trials in patients with advanced hematologic malignancies as monotherapy or in combination with certain combination therapies, such as antifungals.
Two clinical trials against COVID-19 were conducted in 2021, both based on the same cationic peptide dendritic structure containing lysine as branch elements, named KK-46, the structure of which is shown in Figure 3.
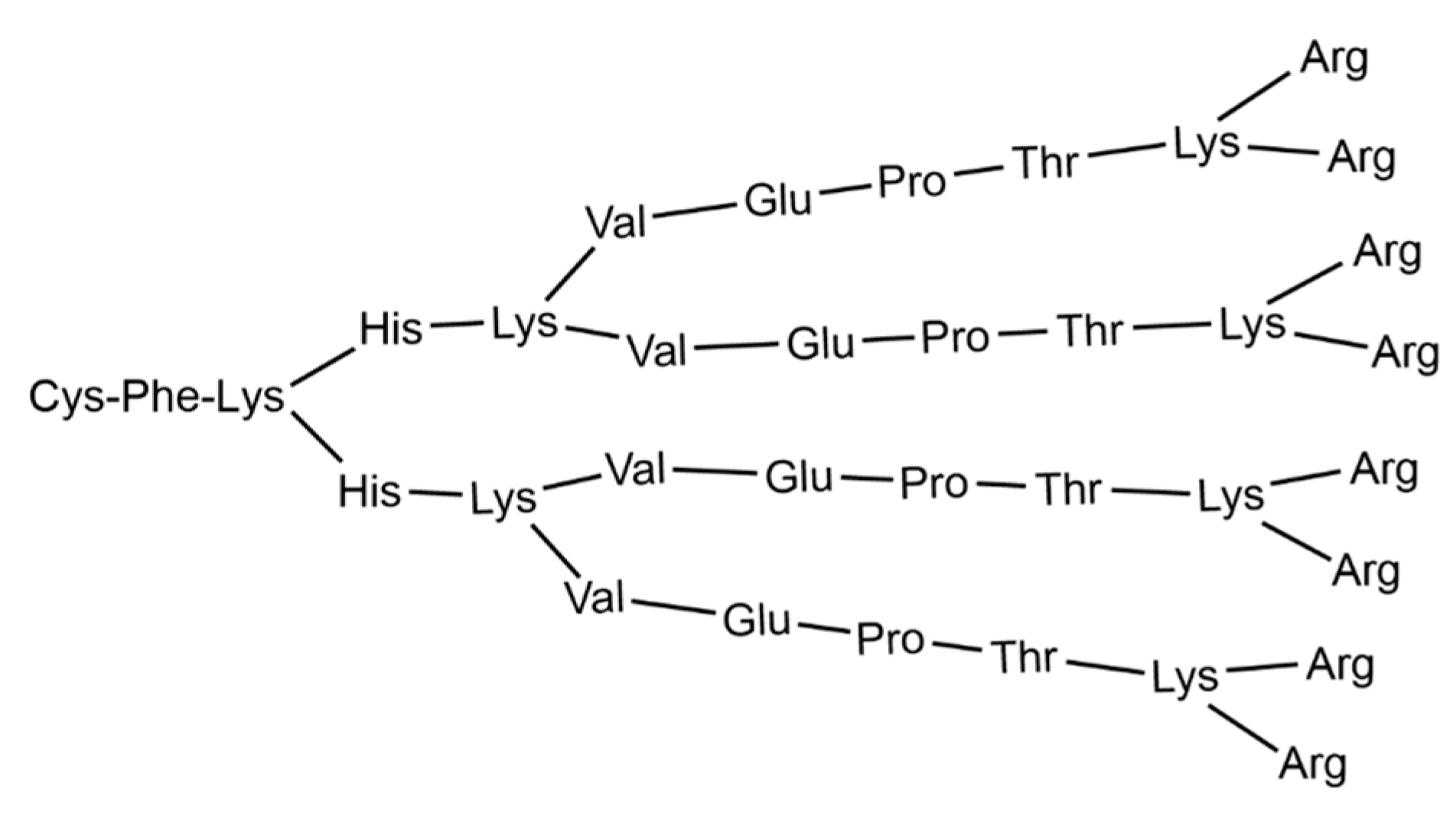
Figure 3. Structure of the cationic peptide dendrimer KK-46, usable as carrier of siCoV [107].
This compound, developed by the Institute of Immunology of the Federal Medical and Biological Agency of Russia, in collaboration with the St. Petersburg Research Institute of Vaccines and Serums, is used as a carrier of siRNA, modified to suppress SARS-CoV-2 (siCoV) by inhibiting its replication [108]. The association of KK-46 with siCoV has been named MIR 19® [109]. Based on preclinical data, the researchers hypothesized that SARS-CoV-2 inhibition by siCoV/KK46 could potentially reduce lung inflammation, thereby improving treatment outcomes. According to the test results, the drug was registered and introduced into civil circulation by the Ministry of Health of the Russian Federation on 22 December 2021 LP-007720 as a direct-acting antiviral agent [110].
3. Conclusion
In summary, distinct dendrimer-based constructs have been clinically tested and have been shown to be safe and effective in Phases 1–3, with some studies still ongoing. Table 1 lists dendrimer-based products in clinical trials, according to the Clinictrials.gov database. These examples clearly highlight the growing use of dendrimer-based drug delivery systems in the pharmaceutical industry.
Thus, dendrimers represent a useful platform for the development of safe vaccines with new properties and application potential and will also be useful for basic research on the mechanisms underlying the induction and control of immunity.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15041106
References
- Caminade, A.-M. Dendrimers, an Emerging Opportunity in Personalized Medicine? J. Pers. Med. 2022, 12, 1334.
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526.
- Nanjwade, B.K.; Bechra, H.M.; Derkar, G.K.; Manvi, F.V.; Nanjwade, V.K. Dendrimers: Emerging polymers for drug-delivery systems. Eur. J. Pharm. Sci. 2009, 38, 185–196.
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959.
- Caminade, A.-M. Inorganic dendrimers: Recent advances for catalysis, nanomaterials, and nanomedicine. Chem. Soc. Rev. 2016, 45, 5174–5186.
- Qiu, J.; Hameau, A.; Shi, X.; Mignani, S.; Majoral, J.P.; Caminade, A.M. Fluorescent Phosphorus Dendrimers: Towards Material and Biological Applications. ChemPlusChem 2019, 84, 1070–1080.
- Apartsin, E.; Caminade, A.-M. Single-Component Physical Hydrogels of Dendritic Molecules. J. Compos. Sci. 2023, 7, 26.
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Ceña, V.; El Kazzouli, S.; Majoral, J.-P. Exploration of biomedical dendrimer space based on in-vitro physicochemical parameters: Key factor analysis (Part 1). Drug Discov. Today 2019, 24, 1176–1183.
- Mignani, S.; Rodrigues, J.; Roy, R.; Shi, X.; Ceña, V.; El Kazzouli, S.; Majoral, J.-P. Exploration of biomedical dendrimer space based on in-vivo physicochemical parameters: Key factor analysis (Part 2). Drug Discov. Today 2019, 24, 1184–1192.
- Maysinger, D.; Zhang, Q.; Kakkar, A. Dendrimers as Modulators of Brain Cells. Molecules 2020, 25, 4489.
- Mignani, S.; Shi, X.; Ceña, V.; Shcharbin, D.; Bryszewska, M.; Majoral, J.-P. In vivo therapeutic applications of phosphorus dendrimers: State of the art. Drug Discov. Today 2021, 26, 677–689.
- Caminade, A.-M.; Majoral, J.-P. Phosphorus dendrimers functionalised with nitrogen ligands, for catalysis and biology. Dalton Trans. 2019, 48, 7483–7493.
- Dias, A.P.; da Silva Santos, S.; da Silva, J.V.; Parise-Filho, R.; Igne Ferreira, E.; Seoud, O.E.; Giarolla, J. Dendrimers in the context of nanomedicine. Int. J. Pharm. 2020, 573, 118814.
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126.
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91.
- Caminade, A.-M.; Ouali, A.; Laurent, R.; Turrin, C.-O.; Majoral, J.-P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015, 44, 3890–3899.
- Montilla, F.; Galindo, A.; Andrés, R.; Córdoba, M.; de Jesús, E.; Bo, C. Carbosilane Dendrons as Solubilizers of Metal Complexes in Supercritical Carbon Dioxide. Organometallics 2006, 25, 4138–4143.
- Rodríguez, L.-I.; Rossell, O.; Seco, M.; Muller, G. Carbosilane Dendrons Containing a P-Stereogenic Phosphine at the Focal Point. Catalytic Behavior of Their Allylpalladium Complexes in the Asymmetric Hydrovinylation of Styrene. Organometallics 2008, 27, 1328–1333.
- García-Peña, N.G.; Caminade, A.-M.; Ouali, A.; Redón, R.; Turrin, C.-O. Solventless synthesis of Ru(0) composites stabilized with polyphosphorhydrazone (PPH) dendrons and their use in catalysis. RSC Adv. 2016, 6, 64557–64567.
- Michlewska, S.; Ionov, M.; Shcharbin, D.; Maroto-Díaz, M.; Ramirez, R.G.; de la Mata, F.J.; Bryszewska, M. Ruthenium metallodendrimers with anticancer potential in an acute promyelocytic leukemia cell line (HL60). Eur. Polym. J. 2017, 87, 39–47.
- Fernandez, J.; Acosta, G.; Pulido, D.; Malý, M.; Copa-Patiño, J.L.; Soliveri, J.; Royo, M.; Gómez, R.; Albericio, F.; Ortega, P.; et al. Carbosilane Dendron–Peptide Nanoconjugates as Antimicrobial Agents. Mol. Pharm. 2019, 16, 2661–2674.
- Gutierrez-Ulloa, C.E.; Sepúlveda-Crespo, D.; García-Broncano, P.; Malý, M.; Muñoz-Fernández, M.A.; de la Mata, F.J.; Gómez, R. Synthesis of bow-tie carbosilane dendrimers and their HIV antiviral capacity: A comparison of the dendritic topology on the biological process. Eur. Polym. J. 2019, 119, 200–212.
- Pędziwiatr-Werbicka, E.; Gorzkiewicz, M.; Horodecka, K.; Abashkin, V.; Klajnert-Maculewicz, B.; Peña-González, C.E.; Sánchez-Nieves, J.; Gómez, R.; de la Mata, F.J.; Bryszewska, M. Silver Nanoparticles Surface-Modified with Carbosilane Dendrons as Carriers of Anticancer siRNA. Int. J. Mol. Sci. 2020, 21, 4647.
- Apartsin, E.K.; Knauer, N.; Kahlert, U.D.; Caminade, A.-M. Amphiphilic Triazine-Phosphorus Metallodendrons Possessing Anti-Cancer Stem Cell Activity. Pharmaceutics 2022, 14, 393.
- Ramos, E.; Davin, L.; Angurell, I.; Ledesma, C.; Llorca, J. Improved Stability of Pd/Al2O3 Prepared from Palladium Nanoparticles Protected with Carbosilane Dendrons in the Dimethyl Ether Steam Reforming Reaction. ChemCatChem 2015, 7, 2179–2187.
- González-García, E.; Gutiérrez Ulloa, C.E.; de la Mata, F.J.; Marina, M.L.; García, M.C. Sulfonate-terminated carbosilane dendron-coated nanotubes: A greener point of view in protein sample preparation. Anal. Bioanal. Chem. 2017, 409, 5337–5348.
- Vásquez-Villanueva, R.; Peña-González, C.E.; Sánchez-Nieves, J.; de la Mata, F.J.; Marina, M.L.; García, M.C. Gold nanoparticles coated with carbosilane dendrons in protein sample preparation. Microchim. Acta 2019, 186, 508.
- Barrios-Gumiel, A.; Sepúlveda-Crespo, D.; Jiménez, J.L.; Gómez, R.; Muñoz-Fernández, M.Á.; de la Mata, F.J. Dendronized magnetic nanoparticles for HIV-1 capture and rapid diagnostic. Colloid Surf. B-Biointerfaces 2019, 181, 360–368.
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Valiente, M.; Gómez, R. Amphiphilic carbosilane dendrons as a novel synthetic platform toward micelle formation. Org. Biomol. Chem. 2017, 15, 7352–7364.
- Xiao, Z.; Cai, C.; Mayeux, A.; Milenkovic, A. The First Organosiloxane Thin Films Derived from SiCl3-Terminated Dendrons. Thickness-Dependent Nano-and Mesoscopic Structures of the Films Deposited on Mica by Spin-Coating. Langmuir 2002, 18, 7728–7739.
- Zhang, Q.; Archer, L.A. Step-Growth Synthesis and Interfacial Friction Properties of Surface Dendron Coatings. Langmuir 2006, 22, 717–722.
- Peterca, M.; Imam, M.R.; Ahn, C.-H.; Balagurusamy, V.S.K.; Wilson, D.A.; Rosen, B.M.; Percec, V. Transfer, Amplification, and Inversion of Helical Chirality Mediated by Concerted Interactions of C3-Supramolecular Dendrimers. J. Am. Chem. Soc. 2011, 133, 2311–2328.
- Zhang, S.; Sun, H.-J.; Hughes, A.D.; Moussodia, R.-O.; Bertin, A.; Chen, Y.; Pochan, D.J.; Heiney, P.A.; Klein, M.L.; Percec, V. Self-assembly of amphiphilic Janus dendrimers into uniform onion-like dendrimersomes with predictable size and number of bilayers. Proc. Natl. Acad. Sci. USA 2014, 111, 9058–9063.
- Apartsin, E.; Caminade, A.M. Supramolecular Self-Associations of Amphiphilic Dendrons and Their Properties. Chem. Eur. J. 2021, 27, 17976–17998.
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983.
- Apartsin, E.; Knauer, N.; Arkhipova, V.; Pashkina, E.; Aktanova, A.; Poletaeva, J.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R. pH-Sensitive Dendrimersomes of Hybrid Triazine-Carbosilane Dendritic Amphiphiles-Smart Vehicles for Drug Delivery. Nanomaterials 2020, 10, 1899.
- Sztandera, K.; Gorzkiewicz, M.; Bątal, M.; Arkhipova, V.; Knauer, N.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R.; Apartsin, E.; Klajnert-Maculewicz, B. Triazine–Carbosilane Dendrimersomes Enhance Cellular Uptake and Phototoxic Activity of Rose Bengal in Basal Cell Skin Carcinoma Cells. Int. J. Nanomed. 2022, 17, 1139–1154.
- Yu, T.; Liu, X.; Bolcato-Bellemin, A.L.; Wang, Y.; Liu, C.; Erbacher, P.; Qu, F.; Rocchi, P.; Behr, J.P.; Peng, L. An Amphiphilic Dendrimer for Effective Delivery of Small Interfering RNA and Gene Silencing In Vitro and In Vivo. Angew. Chem. Int. Edit. 2012, 51, 8478–8484.
- Liu, X.; Zhou, J.; Yu, T.; Chen, C.; Cheng, Q.; Sengupta, K.; Huang, Y.; Li, H.; Liu, C.; Wang, Y.; et al. Adaptive Amphiphilic Dendrimer-Based Nanoassemblies as Robust and Versatile siRNA Delivery Systems. Angew. Chem. Int. Edit. 2014, 53, 11822–11827.
- Liu, X.; Wang, Y.; Chen, C.; Tintaru, A.; Cao, Y.; Liu, J.; Ziarelli, F.; Tang, J.; Guo, H.; Rosas, R.; et al. A Fluorinated Bola-Amphiphilic Dendrimer for On-Demand Delivery of siRNA, via Specific Response to Reactive Oxygen Species. Adv. Funct. Mater. 2016, 26, 8594–8603.
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and Their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631.
- Torre, P.; Xiao, Q.; Buzzacchera, I.; Sherman, S.E.; Rahimi, K.; Kostina, N.Y.; Rodriguez-Emmenegger, C.; Möller, M.; Wilson, C.J.; Klein, M.L.; et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385.
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532.
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymenrs–starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132.
- Newkome, G.R.; Yao, Z.Q.; Baker, G.R.; Gupta, V.K. Micelles. 1 Cascade molecules—A new approach to micelles—A -arborol. J. Org. Chem. 1985, 50, 2003–2004.
- Wooley, K.L.; Hawker, C.J.; Frechet, J.M.J. Hyperbranched macromolecules via a novel double-stage convergent growth approach. J. Am. Chem. Soc. 1991, 113, 4252–4261.
- Zhou, L.L.; Roovers, J. Synthesis of novel carbosilane dendritic macromolecules. Macromolecules 1993, 26, 963–968.
- de Brabander van den Berg, E.M.M.; Meijer, E.W. Poly(Propylene Imine) Dendrimers—Large-Scale Synthesis by Hetereogeneously Catalyzed Hydrogenations. Angew. Chem. Int. Edit. 1993, 32, 1308–1311.
- Percec, V.; Mitchell, C.M.; Cho, W.D.; Uchida, S.; Glodde, M.; Ungar, G.; Zeng, X.B.; Liu, Y.S.; Balagurusamy, V.S.K.; Heiney, P.A. Designing libraries of first generation AB(3) and AB(2) self-assembling dendrons via the primary structure generated from combinations of (AB)(y)-AB(3) and (AB)(y)-AB(2) building blocks. J. Am. Chem. Soc. 2004, 126, 6078–6094.
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617.
- Newkome, G.R.; Moorefield, C.N.; Chakraborty, S. A Long Pathway to the Quantitative Assembly of Metallodendrimers. J. Inorg. Organomet. Polym. Mater. 2018, 28, 360–368.
- Tomalia, D.A.; Nixon, L.S.; Hedstrand, D.M. The Role of Branch Cell Symmetry and Other Critical Nanoscale Design Parameters in the Determination of Dendrimer Encapsulation Properties. Biomolecules 2020, 10, 642.
- Zhang, D.P.; Atochina-Vasserman, E.N.; Lu, J.C.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.P.; et al. The Unexpected Importance of the Primary Structure of theHydrophobic Part of One-Component Ionizable Amphiphilic JanusDendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753.
- De la Mata, F.J.; Gómez, R.; Cano, J.; Sánchez-Nieves, J.; Ortega, P.; Gallego, S.G. Carbosilane dendritic nanostructures, highly versatile platforms for pharmaceutical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, e1871.
- Caminade, A.-M. Phosphorus Dendrimers as Nanotools against Cancers. Molecules 2020, 25, 3333.
- Majoral, J.-P.; Zablocka, M.; Ciepluch, K.; Milowska, K.; Bryszewska, M.; Shcharbin, D.; Katir, N.; El Kadib, A.; Caminade, A.-M.; Mignani, S. Hybrid phosphorus–viologen dendrimers as new soft nanoparticles: Design and properties. Org. Chem. Front. 2021, 8, 4607–4622.
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-responsive dendrimers in drug delivery. Biomater. Sci. 2016, 4, 375–390.
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12.
- Hermanson, G.T. (Ed.) Bioconjugate Techniques, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008; p. 1195.
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- SPL7013 Gel—Male Tolerance Study. Available online: https://clinicaltrials.gov/ct2/show/NCT00370357 (accessed on 29 January 2023).
- VivaGel™ in Healthy Young Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00331032 (accessed on 29 January 2023).
- Safety and Acceptability of SPL7013 Gel (VivaGel™) in Sexually Active Women. Available online: https://clinicaltrials.gov/ct2/show/NCT00442910 (accessed on 29 January 2023).
- Retention and Duration of Activity of SPL7013 (VivaGel®) after Vaginal Dosing. Available online: https://clinicaltrials.gov/ct2/show/NCT00740584 (accessed on 29 January 2023).
- Dose Ranging Study of SPL7013 Gel for Treatment of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT01201057 (accessed on 29 January 2023).
- Dose-ranging Study of SPL7013 Gel for the Prevention of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT01437722 (accessed on 29 January 2023).
- A Phase 3 Study of SPL7013 Gel (VivaGel) for the Treatment of Bacterial Vaginosis. Available online: https://clinicaltrials.gov/ct2/show/NCT01577537 (accessed on 29 January 2023).
- Efficacy and Safety Study of SPL7013 Gel to Prevent the Recurrence of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT02236156 (accessed on 29 January 2023).
- Efficacy and Safety Study of SPL7013 Gel to Prevent the Recurrence of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT02237950 (accessed on 29 January 2023).
- A Study of AZD0466 in Patients With Advanced Hematologic or Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04214093 (accessed on 17 March 2023).
- A Phase I/II Study of AZD0466 as Monotherapy or in Combination With Anticancer Agents in Advanced Non-Hodgkin Lymphoma. Available online: https://clinicaltrials.gov/ct2/show/NCT05205161 (accessed on 29 January 2023).
- Partnered-DEP® products—AZD0466. Available online: https://starpharma.com/drug_delivery/dep-azd0466 (accessed on 29 January 2023).
- Study of AZD0466 Monotherapy or in Combination in Patients With Advanced Haematological Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT04865419 (accessed on 29 January 2023).
- Treatment of Non-responding to Conventional Therapy Inoperable Liver Cancers by In Situ Introduction of ImDendrim (ImDendrim). Available online: https://clinicaltrials.gov/ct2/show/NCT03255343 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of OP-101 after Intravenous Administration in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03500627 (accessed on 29 January 2023).
- A Clinical Study to Measure the Effect of OP-101 after Being Administered Subcutaneous in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT04321980 (accessed on 29 January 2023).
- A Study to Evaluate OP-101 (Dendrimer N-acetyl-cysteine) in Severe Coronavirus Disease 2019 (COVID-19) Patients (PRANA). Available online: https://clinicaltrials.gov/ct2/show/NCT04458298 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of D-4517.2 after Subcutaneous Administration in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT05105607 (accessed on 29 January 2023).
- A Study to Evaluate the Safety, Tolerability and Pharmacokinetics of D-4517.2 after Subcutaneous Administration in Subjects With Neovascular (Wet) Age-Related Macular Degeneration (AMD) or Subjects With Diabetic Macular Edema (DME) (Tejas). Available online: https://clinicaltrials.gov/ct2/show/NCT05387837 (accessed on 29 January 2023).
- The siCoV/KK46 Drug Open-safety Study. Available online: https://clinicaltrials.gov/ct2/show/NCT05208996 (accessed on 29 January 2023).
- Evaluation of Safety & Efficacy of MIR 19 ® Inhalation Solution in Patients With Moderate COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT05184127 (accessed on 29 January 2023).
- Holmes, W.R.; Maher, L.; Rosenthal, S.L. Attitudes of men in an Australian male tolerance study towards microbicide use. Sex Health 2008, 5, 273–278.
- Rosenthal, S.L.; Holmes, W.; Maher, L. Australian men’s experiences during a microbicide male tolerance study. Aids Care Psychol. Socio Med. Asp. Aids/HIV 2009, 21, 125–130.
- Chen, M.Y.; Millwood, I.Y.; Wand, H.; Poynten, M.; Law, M.; Kaldor, J.M.; Wesselingh, S.; Price, C.F.; Clark, L.J.; Paull, J.R.A.; et al. A Randomized Controlled Trial of the Safety of Candidate Microbicide SPL7013 Gel When Applied to the Penis. Jaids 2009, 50, 375–380.
- Cohen, C.R.; Brown, J.; Moscicki, A.B.; Bukusi, E.A.; Paull, J.R.A.; Price, C.F.; Shiboski, S. A Phase I Randomized Placebo Controlled Trial of the Safety of 3% SPL7013 Gel (VivaGel (R)) in Healthy Young Women Administered Twice Daily for 14 Days. PLoS ONE 2011, 6, e16285.
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C.; et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). Aids 2011, 25, 1057–1064.
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel (R)) Retains Potent HIV-1 and HSV-2 Inhibitory Activity following Vaginal Administration in Humans. PLoS ONE 2011, 6, e24095.
- Moscicki, A.B.; Kaul, R.; Ma, Y.F.; Scott, M.E.; Scott, M.E.; DAUD, I.I.; Bukusi, E.A.; Shiboski, S.; Rebbapragada, A.; Huibner, S.; et al. Measurement of Mucosal Biomarkers in a Phase 1 Trial of Intravaginal 3% StarPharma LTD 7013 Gel (VivaGel) to Assess Expanded Safety. Jaids 2012, 59, 134–140.
- Carballo-Dieguez, A.; Giguere, R.; Dolezal, C.; Chen, B.A.; Kahn, J.; Zimet, G.; Mabragana, M.; Leu, C.S.; McGowan, I. “Tell Juliana”: Acceptability of the Candidate Microbicide VivaGel(A (R)) and Two Placebo Gels Among Ethnically Diverse, Sexually Active Young Women Participating in a Phase 1 Microbicide Study. AIDS Behav. 2012, 16, 1761–1774.
- Waldbaum, A.S.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Edmondson, S.R.; Castellarnau, A.; McCloud, P.; Kinghorn, G.R. A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis. PLoS ONE 2020, 15, e0232394.
- Chavoustie, S.E.; Carter, B.A.; Waldbaum, A.S.; Donders, G.G.G.; Peters, K.H.; Schwebke, J.R.; Paull, J.R.A.; Price, C.F.; Castellarnau, A.; McCloud, P.; et al. Two phase 3, double-blind, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 245, 13–18.
- Australian New Zealand Clinical Trials Registry. Available online: https://anzctr.org.au/ (accessed on 17 March 2023).
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gibbons, F.D.; et al. Design and optimisation of dendrimer-conjugated Bcl-2/x(L) inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112.
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122.
- Feeney, O.M.; Ardipradja, K.; Noi, K.F.; Mehta, D.; De Rose, R.; Yuen, D.; Johnston, A.P.R.; Kingston, L.; Ericsson, C.; Elmore, C.S.; et al. Subcutaneous delivery of a dendrimer-BH3 mimetic improves lymphatic uptake and survival in lymphoma. J. Control. Release 2022, 348, 420–430.
- Akhtar, N.; Ashford, M.B.; Beer, L.; Bowes, A.; Bristow, T.; Broo, A.; Buttar, D.; Coombes, S.; Cross, R.; Eriksson, E.; et al. The Global Characterisation of a Drug-Dendrimer Conjugate—PEGylated poly-lysine Dendrimer. J. Pharm. Sci. 2022, 112, 844–858.
- Yang, G.; Sadeg, N.; Tahar, H.B. New Potential In Situ Anticancer Agent Derived from rhenium Nitro-Imidazole Ligand Loaded 5th Generation Poly-L-Lysine Dendrimer for Treatment of Transplanted Human Liver Carcinoma in Nude Mice. Drug Design. 2017, 06, 1–7.
- Tomalia, D.A.; Naylor, A.M.; Goddard, W.A. STARBURST Dendrimers: Molecular Level Control of Size, Shape, Surface Chemistry, Topology and Flexibility from Atoms to Macroscopic Matter. Angew. Chem. Int. Edit. 1990, 29, 138–175.
- Tomalia, D.A. Dendrimer research. Science 1991, 252, 1231–1232.
- Tomalia, D.A. In quest of a systematic framework for unifying and defining nanoscience. J. Nanopart. Res. 2009, 11, 1251–1310.
- Stanwix, H.; Tomalia, D.A. An architectural journey: From trees, dendrons/dendrimers to nanomedicine. Nanomedicine 2012, 7, 953–956.
- Tomalia, D.A.; Christensen, J.B.; Boas, U. Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Applications, and the Future; Cambridge University Press: Cambridge, UK, 2012; pp. 1–412.
- Kukowska-Latallo, J.F.; Bielinska, A.U.; Johnson, J.; Spindler, R.; Tomalia, D.A.; Baker, J.R. Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc. Natl. Acad. Sci. USA 1996, 93, 4897–4902.
- McCarthy, T.D.; Karellas, P.; Henderson, S.A.; Giannis, M.; O’Keefe, D.F.; Heery, G.; Paull, J.R.A.; Matthews, B.R.; Holan, G. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005, 2, 312–318.
- Gusdon, A.M.; Faraday, N.; Aita, J.S.; Kumar, S.; Mehta, I.; Choi, H.A.; Cleland, J.L.; Robinson, K.; McCullough, L.D.; Ng, D.K.; et al. Dendrimer nanotherapy for severe COVID-19 attenuates inflammation and neurological injury markers and improves outcomes in a phase2a clinical trial. Sci. Transl. Med. 2022, 14, eabo2652.
- Ashvattha Therapeutics. Available online: https://avttx.com/pipeline/ophthalmology/ (accessed on 17 March 2023).
- Khaitov, M.R.; Shilovskii, I.P.; Kozhikhova, K.V.; Kofiadi, I.A.; Smirnov, V.V.; Koloskova, O.O.; Sergeev, I.V.; Trofimov, D.Y.; Trukhin, V.P.; Skvortsova, V.I. Combination Antiviral Formulation against SARS-CoV-2 Comprising SARS-CoV-2 Genome-Targeting siRNAs and Transfection-Enhancing Cationic Peptide Dendrimer. RU2746362 C1, 11 March 2021.
- Khaitov, M.; Nikonova, A.; Shilovskiy, I.; Kozhikhova, K.; Kofiadi, I.; Vishnyakova, L.; Nikolskii, A.; Gattinger, P.; Kovchina, V.; Barvinskaia, E.; et al. Silencing of SARS-CoV-2 with modified siRNA-peptide dendrimer formulation. Allergy 2021, 76, 2840–2854.
- Khaitov, M.; Nikonova, A.; Kofiadi, I.; Shilovskiy, I.; Smirnov, V.; Elisytina, O.; Maerle, A.; Shatilov, A.; Shatilova, A.; Andreev, S.; et al. Treatment of COVID-19 patients with a SARS-CoV-2-specific siRNA-peptide dendrimer formulation. Allergy 2023, 1–15.
- Registration Certificate LP-007720. Available online: https://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=bb62a3b8-7b38-4d71-aa9b-51660813a32a (accessed on 17 March 2023).
