1. Vaccines
Vaccines have been introduced to prevent several childhood diseases and eradicate some, such as smallpox and rinderpest [
18]. Edward Jenner is considered the pioneer of vaccination for developing the vaccine for the smallpox virus but the same principle was used by Louis Pasteur for other pathogens including rabies virus and fowl cholera [
41,
42,
43]. Vaccination fights against AMR both directly, by reducing the incidence of bacterial infections, and indirectly, via the various ways in which they can reduce the use of antibiotics [
25]. Furthermore, vaccines can reduce both the inappropriate use of antibiotics prescribed in case of viral infections (such as influenza) and the need for antibiotic treatment to cure secondary bacterial infections [
18,
25].
Vaccines differ from antibiotics because they target several immunogenic epitopes and are often used to prevent infections [
18,
44]. When used prophylactically, vaccines train the immune system to recognize and effectively respond to future infections by preventing their occurrence or reducing their severity [
18,
44]. When the majority of the population is vaccinated, the remainder is protected via herd immunity [
45]. The reduced use of antimicrobials slows the pressure of developing resistance by pathogens and normal flora [
25,
27]. This leads to limited development of MDR pathogens and associated complications. Therefore, a drop in vaccination can be disastrous among nonvaccinated people protected via herd immunity and lead to recurrent epidemics.
Vaccination of children allows them to grow well until becoming adults by reducing morbidity and mortality. It is estimated that every dollar spent on vaccine production brings an economic return of 44 USD [
43]. Rosini et al. [
18] highlighted the importance of vaccines in increasing people’s life expectancy by decreasing mortality rates. Vaccination has been associated with increasing national economic growth and poverty reduction [
46]. The economic gains of vaccination can be seen in terms of reduced treatment and healthcare costs, productivity gains where healthy children demonstrate improved educational attainment at school through increased attendance and better cognitive performance, and minimizing impact on families as sick people require a caregiver, mainly a parent, to spend time and money [
47]. Despite the facts on the efficacy of vaccines in disease prevention, reducing mortality rates, and improving the economy, their contribution to addressing the threat of AMR is underappreciated [
27,
48].
The WHO report on the pipeline for vaccines targeting bacterial priority pathogens showed that 61 candidates are at different levels of clinical development [
49]. Resistance to commonly used antimicrobials has been reported in SSA. For instance, a study by Musa et al. [
50] reported a pooled prevalence of 2.1% (95% CI; 1.7–2.5%) for multidrug-resistant tuberculosis (MDR-TB) in new cases. Hlashwayo et al. [
51] reported that 30% of
Campylobacter isolates were resistant to ciprofloxacin. Of the 12 priority pathogens [
52], licensed vaccines exist for only four species: pneumococcal disease (
Streptococcus pneumoniae), Hib
(Haemophilus influenzae type b) Tuberculosis (
Mycobacterium tuberculosis), and Typhoid fever (
Salmonella typhi) [
49]. The same report highlighted that, for extraintestinal pathogenic
Escherichia coli (ExPEC),
Salmonella enterica ser.
Paratyphi A,
Neisseria gonorrhoeae, and
Clostridioides difficile, vaccines are in late-stage clinical trials with high development feasibility.
Although vaccines against a number of pathogens are available, there is a shortage of vaccines against infectious diseases including, malaria, tuberculosis, and HIV-1 [
43]. The development of vaccines against enterotoxigenic
E. coli (ETEC),
Klebsiella pneumoniae, nontyphoidal
Salmonella (NTS),
Campylobacter spp., and
Shigella spp. will not yield positive results in the near future [
49]. The lack of vaccine for a number of pathogens has been associated with different reasons, including (i) having several antigens making the selection of a vaccine target very challenging, (ii) difficulty in generating a memory (B and T-cells) response for antigenically diverse pathogens, and (iii) difficulty in finding suitable and effective animal models. Moreover, vaccine development is costly and a vaccine candidate to reach the market should pass through lengthy regulatory processes, limiting the investment by pharmaceutical companies. Therefore, efforts in financing vaccine development against various AMR pathogens should be encouraged.
The SSA is known to have high birth rates, as well as high childhood death rates, partly attributable to a disproportionate lack of vaccines for preventable infections [
53]. Although there is progress in immunization, Africa still lags behind in terms of introducing new vaccines and eliminating vaccine-preventable diseases (VPDs) [
54]. It is reported that 50% of the world’s unvaccinated or under-vaccinated children are in Africa [
55]. In SSA, immunization delays also compromise efforts to prevent morbidity and mortality associated with infectious diseases. For instance, 63% of children in SSA missed the first dose of the measles vaccines (at 9 months), and the majority of these cases were reported from mothers with limited literacy [
56]. Another study reported that the BCG vaccine had the highest coverage at 86.2% (95% CI: 85.6% to 86.8%); whereas three doses of oral polio vaccine had the lowest coverage at 68.2% (95% CI: 67.5% to 68.9%) [
57]. In addition to education status, the distance of households from clinical settings, the vaccine supply chain, poverty, and training of healthcare workers have been highlighted as factors contributing to incomplete immunization [
53,
54].
Efforts toward equitable access to vaccines are needed to reduce both the morbidity and the mortality of VPDs. It is assumed that Africa consumes 25% of the world’s vaccines but only 1% of them are manufactured in Africa [
58]. This creates a dependency on external support and hampers the control of infectious diseases. However, efforts to reverse the trend are ongoing, and there is a target to reach production on the continent for 60% of vaccines needed by Africans [
58]. Initiatives of various pharmaceutical companies to establish vaccine manufacturing plants in SSA will boost immunization coverage and reduce the cost of production, as such vaccines will be manufactured on the African continent.
2. Education and Awareness
One way to reduce the burden due to AMR is through promoting its awareness and designing curricula/teaching materials for different categories of people [
59]. Education can focus on antibiotic misuse, behavioral change, and the advantages of wise use of antimicrobials.
Healthcare professionals are at the frontline in the fight against AMR. However, the lack of teaching materials or specific modules hampers efforts to control and contain the AMR threat [
60]. Training should start with a review of the curricula to integrate AMR topics. A study in the US showed that the majority of medical students learned about antibiogram during clinical placements, and 73% of them suggested formal training on antibiogram interpretation [
61]. A study conducted in the East Africa showed that final-year medical and pharmacy students have limited knowledge of both AMR and antibiotic use in clinical scenarios [
62]. Moreover, the awareness of AMR threat was reported to be limited even among healthcare students in Rwanda [
12,
63]. Nisabwe et al. [
12] reported that 96% and 83% of dental and pharmacy students heard about AMR from non-curricula sources and were unfamiliar with antimicrobial stewardship, respectively. In LMICs, this is worrying as final-year medical and pharmacy students are involved in prescribing or dispensing antimicrobials due to a shortage of qualified personnel [
63]. Furthermore, the full potential of pharmacists is not exploited considering that they are mainly used in stocking and supplying drugs [
64]. There is a need to remind prescribers and pharmacists about their role in educating patients on the importance of completing the prescribed antimicrobials. Curricula for healthcare professionals need revisions to include special modules on AMR or provide specific extracurricular training. Medical or health professional councils are recommended to organize training sessions for their members on AMR. To motivate the enrolment of members, such training can be counted as continued professional development (CPD), often required to renew members’ licenses.
To address the AMR threat, it is important to stimulate behavioral change among different categories of the society. Studies have reported higher AMR where the level of public awareness was low [
65,
66]. The training about AMR should not be restricted to healthcare professionals because everyone has a role to play. Engaging the community about the appropriate use of antimicrobials can help preserve the limited arsenal of existing antimicrobials [
25,
67]. Public engagement has proven to be effective in tackling the AMR problem [
68] via the use of healthcare professionals, social media, and apps [
69,
70]. A study conducted in Cyprus showed that 72.3% of the respondents were informed about AMR from healthcare professionals or social media [
70]. However, social media, television, and newspapers can be damaging because wrong information about AMR can also be shared. Therefore, information on AMR from social media should be handled with care in case it is from untrusted sources.
Training sessions proved to be effective in increasing the knowledge about AMR. A study in Tanzania showed that the AMR-related knowledge among secondary school students was 37% but increased to 90% after getting training on AMR [
66]. Through community engagement, the community takes ownership of the issue and seeks solutions that are specific to the settings [
67]. Experience has shown that media campaigns are more effective than healthcare professionals for raising awareness of antibiotics but healthcare professionals are known to influence behavioral change [
71]. The behavioral change by healthcare personnel is thought to be associated with physical contact with the community and being an expert in the field [
67]. In addition to the general public, it is crucial to educate and engage patients on AMR. Patients should be taught about adhering to prescriptions and warned of the possible consequences of not taking antimicrobials as prescribed by the clinician [
59]. However, In LMICs, doctors are overwhelmed due to the high patient–doctor ratio affecting the time spent on patients at clinical settings [
72]. Everyone should be taught about the safe disposal of expired, unused, or unwanted antimicrobials, considering that mishandling of drugs contributes to the spread of AMR threat.
The problem of AMR goes beyond human medicine and also touches veterinary medicine and the environment. The burden of AMR is expected to reduce livestock production by approximately 11% of the yield by 2050, with a particularly severe impact on LMICs [
73]. Antimicrobials have been used in animals for therapy, growth promotion, and prophylaxis [
4,
15]. Additionally, some antimicrobials (erythromycin, ampicillin, and colistin) are used in both people and animals. Antimicrobials or antimicrobial residues from humans and animals or animal products end up in the environment, affecting aquatic lives [
74]. Furthermore, livestock waste and manure are used as fertilizers yet carry many zoonotic pathogens, including MDR ones. Therefore, farmers and the community at large should be involved in awareness and education programs aimed at the proper use of existing antimicrobials and the management of livestock waste that can spread AMR pathogens.
3. Infection Prevention and Control by Improving WASH
Healthcare facilities are crucial in reducing disease, but the lack of basic WASH services contributes to more infections, extended hospitalizations, and preventable deaths [
75]. Infection prevention and control (IPC) by improving WASH is one of five objectives in the World Health Organization’s (WHO) Global Action Plan on AMR [
76]. The WHO and the United Nations Children’s Fund (UNICEF), through the Joint Monitoring Program (JMP), are responsible for global monitoring of the Sustainable Development Goal (SDG) targets for WASH [
77]. WASH is essential on battlefronts for reducing and controlling the rising of AMR. Poor sanitation and hygiene lead to infections that promote extensive use of antimicrobials. Providing WASH and improving wastewater systems will lead to a major decline in infection and AMR pathogens around the world [
78,
79]. The lack of WASH may account for much of the AMR spread in LMICs and beyond health facilities [
80].
Wastewater and sludge from animals, humans, and plants are overloaded with AMR pathogens and antimicrobial residues that contaminate water bodies [
81]. This could result in the widespread of AMR microorganisms, thus increasing the risk of human exposure to AMR pathogens. The effectiveness of hygiene has a significant impact on lowering infection rates and the need for antimicrobial treatment, thus reducing the spread and development of AMR [
82]. A previous study conducted in Guatemala showed that improved hygiene was associated with a 30–50% decrease in the odds of detecting resistance to all antimicrobials [
83]. Ramay et al. [
83] found that, for nine out of 10 antibiotics, the odds of detecting resistant bacteria decreased by ~32% (odds ratios, OR 0.53–0.8,
p < 0.001) for every unit of improvement on a hygiene scale. Moreover, this study highlighted that differences in hygiene practices between households significantly impacted the AMR rate associated with antimicrobial use. Thus, understanding and addressing the role of WASH in combatting AMR is a critical element to be included in a One Health approach. Therefore, improving the quality of drinking water, sanitation systems, and treatment of wastewater and sludge are needed.
4. Natural Products
Currently, available treatments are failing to treat some of the microbial infections due to increasing reports of AMR [
85]. Biofilms form a layer that prevents the entry of antimicrobials, thus making them ineffective [
86]. Considering the problems associated with AMR pathogens and biofilm, there is an increasing need to develop alternative methods for treating microbial infections [
35,
87]. Worldwide, various alternatives to conventional antimicrobials have been developed, including herbal antimicrobial compounds, antimicrobial peptides, bacteriophages, and nanomaterials. The literature shows that over 80% of modern drugs are manufactured from natural products [
88], and 88% of people in Africa rely on complementary and alternative medicine.
5. One Health Approach
The One Health (OH) approach has historically ingrained the fundamentals of social, medical, and ecological concepts. This approach can be traced back prior to the 21st century, to researchers such as Louis Pasteur, Robert Koch, William Osler, Rudolph Virchow, and others who sought to challenge boundaries across the field of animal and human health [
114]. One Health is defined as an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals, and ecosystems. It recognizes that the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) is closely linked and interdependent. The approach mobilizes multiple sectors, disciplines, and communities at varying levels of society to work together to foster wellbeing and tackle threats to health and ecosystems while addressing the collective need for clean water, energy and air, and safe and nutritious food, taking action on climate change and contributing to sustainable development [
115]. OH has been reconceptualized to manage the health catastrophes caused by accelerating environmental changes, as well as human and animal population growth [
116].
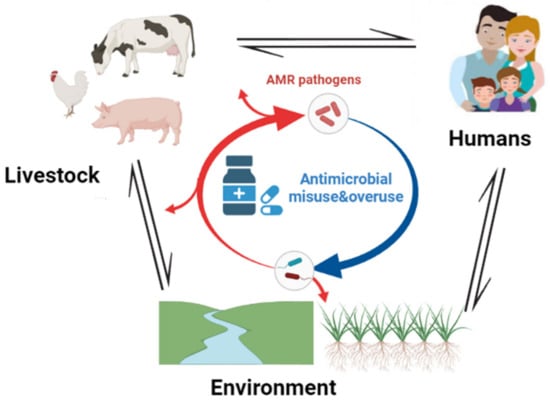
AMR is among the major global health challenges that necessitate an OH approach (
Figure 2). It is known that one of the drivers of AMR is the misuse of antimicrobials in human, animal, and environmental sectors [
117]. Furthermore, today’s AMR issues are complex; there are multifactorial issues that arise in humans, animals, or the environment, as well as food safety. There is a correlation between the occurrence of AMR from human samples and that from farm animals and the wider environment [
118]. Waste from livestock is loaded with AMR pathogens and antimicrobial residues that reach the environment through sewage disposal and agricultural runoff [
23]. To find solutions to such issues from a single discipline, whether medical, veterinary, or ecological, is unlikely to provide sustainable solutions. Therefore, human, animal, and environmental health is interdependent and must be confronted together.
Figure 2. Schematic illustration of the transmission paths of AMR pathogens between animals, humans, and the environment. The image was created using
BioRender.com.
The One Health Global Leaders Group (OH-GLG) on AMR has called for urgent support and prioritization of actions to reduce drug-resistant infections through sustainable and responsible access to and use of antimicrobials. The OH-GLG has also established a tripartite joint secretariat on AMR, including the World Health Organization (WHO), the Food and Agriculture Organization (FAO), and the World Organization for Animal Health (WOAH), emphasizing the importance of seizing the opportunity to use the One Health approach [
119]. To establish an OH approach for tackling AMR, the tripartite became quadripartite after being joined by the United Nations Environment Program (UNEP) [
120]. The quadripartite joint secretariat organized a World Antimicrobial Awareness Week (WAAW), celebrated between 18 and 24 November every year [
6,
121]. The aim of WAAW is to promote awareness and understanding of AMR and to inform of best practices to reduce its emergence and spread. The theme for the year 2022 was “preventing antimicrobial resistance together”’ [
122].
6. AMR Surveillance Program
Disease surveillance is an ongoing systematic collection, analysis, visualization, and interpretation of disease-related data [
131]. In 2015, the WHO launched the Global Antimicrobial Resistance and Use Surveillance System (GLASS), the first global collaborative effort to standardize AMR surveillance. GLASS has enrolled 109 countries and territories worldwide since its inception. The system monitors antimicrobial consumption (AMC) and AMR at the national level. GLASS has been gradually expanding on the basis of the number of covered sites. For example, GLASS recorded data from 24,803 surveillance sites in 70 countries, while only 729 surveillance sites were covered in 2017 [
132]. GLASS keeps track of AMC and AMR in humans, as well as in the food chain and the environment.
In Africa, the joint collaborative effort was established by Africa CDC and formed the Antimicrobial Resistance Surveillance Network (AMRSNET) in 2017. The implemented framework aligns with the WHO GLASS action plan to fight AMR and to improve the detection of antibiotic-resistant pathogens in humans and animals on a continental scale [
133]. Moreover, the network primarily coordinates AMR surveillance, prevention, and control with Ministries of Health and other key partners following the OH model. The global action plan has been adopted in most SSA countries and integrated into the strategies for prevention and combating AMR. However, full implementation of country-specific action plans remains challenged by existing barriers [
134].
Countries such as Tanzania, Ghana, and South Africa are at an advanced level of implementing their NAP for AMR surveillance [
135]. For instance, the NAP in Kenya uses an integrated OH model, and it was jointly endorsed by cabinet secretaries responsible for human health, animal health, and crop production [
135]. Data from Environmental Health and Water Quality monitoring captured by local municipalities also contribute to strategic information in the prevention and control of AMR in South Africa [
136]. In Tanzania, the strategic framework for AMR surveillance is overseen by a National Multisectoral Coordinating Committee (MCC). It also uses the OH model, and it is expected to mitigate AMR and antimicrobial misuse in the human, animal, crop, food, and environment sectors, where priority pathogens have been identified based on prevalence, zoonotic disease, food-borne illness, and potential pathogenicity [
137]. A joint effort of multisectoral stakeholders seen in Ghana formed the AMR Policy Platform, with the governing structure strongly influencing the AMR policy development and national action plan adaptation at the global standard [
138]. Challenges in integrating a multidisciplinary model arise in the absence of coordination effort and having systems operating in silos. However, the adoption of a global action plan for AMR encouraged the effort of the OH model. It also bolstered the standardization of AMR surveillance, prevention, and control in various countries. It is important to mention that countries are scaling up surveillance efforts to tackle AMR through the OH lens in an attempt to implement effective and sustainable interventions [
139].
7. Antimicrobial Stewardship Programs
Antimicrobial stewardship (AMS) programs are hospital-based programs, aiming at promoting the appropriate use of antimicrobials, which proved to be effective in increasing compliance with antibiotic policy and reducing the duration of antimicrobial therapy, cost of antibiotics, AMR, and healthcare-associated infections [
140]. AMS programs also improve patient care and safety [
141], reducing treatment failure rates [
142,
143]. In 2019, the WHO published a practical implementation toolkit for AMS programs in healthcare facilities in LMICs which face a disproportionate burden of antimicrobial resistance, as well as challenges related to resource availability [
144]. A study showed that reducing antimicrobial prescriptions for inpatients significantly improved microbiological and clinical outcomes, as well as reduced rates of AMR pathogens [
145].
There is a shortage of data on the implementation of AMS programs in African countries [
146], but the available data showed that only 12% of respondents in Africa had any form of AMS in place [
147]. Howard et al. [
147] mentioned that respondents were representatives from the hospitals of selected countries, but no information was included about the names of countries involved. Despite a limited number of AMS programs, there has been an improvement in outcome measures (e.g., decrease in antibiotic consumption, compliance with antibiotic policy, and reduction in surgical site infections) where the AMS program was implemented [
140]. A study conducted in South Africa at an academic teaching hospital showed a 19.6% reduction in antibiotic consumption after introducing an AMS program [
148]. Akpan et al. [
140] reported that AMS programs were mainly found in the Southern African region, followed by the East African region. Unfortunately, there were no AMS programs in West Africa, which has the highest number of countries. This highlights the need to sensitize governments in SSA to initiate AMS programs due to their contribution to improving the quality of health and reducing the AMR problem.
8. Functional National Action Plans (NAPs) on AMR
NAP is a nationwide multisectoral document aimed at controlling and combating AMR through a One Health approach including all sectors, especially human health, animal health, and the environment, in order to contribute to the welfare of the population, as well as contribute to global health security. The plan requires a coordinated response to address its complex, multifaceted nature. In May 2015, 194 member states of the WHO endorsed the Global Action Plan (GAP) on AMR and committed to developing and integrating the five objectives and corresponding actions of the GAP into national action plans (NAPs) to address AMR [
149,
150]. As of October 2021, 148 countries globally have finalized their NAPs that are consistent with the objectives of the GAP. Furthermore, 38 (81%) out of 47 countries in the WHO African region have developed NAPs, of which 29 are approved by their national authorities [
151]. In several LMICs, implementation of NAPs for AMR remains fragmented and lacks an OH approach [
152].
9. Political Will/Governance/Regulations
AMR is a multisectoral issue requiring diverse actors to collaborate across issue areas and national borders. This is reflected in the GAP on AMR, which brings together the WHO, the WOAH, the FAO, and the UNEP in an unprecedented attempt to build an effective AMR global governance apparatus based on a holistic “One Health” approach [
149].
At the country level, governance mechanisms are critical for effective intersectoral coordination of activities to combat AMR. They are also important while engaging key stakeholders in developing and implementing NAPs. Governance mechanisms need political support and authority to take action. Governance mechanisms are more likely to be effective if terms of reference are clearly defined. Under the fourth objective of GAP, which is to optimize the use of antimicrobial medicines in human and animal health, countries are urged to put in place effective and enforceable regulations and governance for licensing, distribution, use, and quality assurance of antimicrobial medicines in human and animal health. Such regulations also include a regulatory framework for the preservation of new antimicrobials. This will enable us to tackle the AMR threat successfully while ensuring access to appropriate and affordable antimicrobial treatment in LMICs [
149].
The International Health Regulations (IHR) 2005 and the Good Manufacturing Practice (GMP) offer additional global regulatory tools to address AMR. The IHR provides a legally binding framework for 194 State Parties on how to report and contain public health threats that have the potential to cross borders. They can contribute to the response to emerging AMR threats such as carbapenem-resistant Enterobacteriaceae (CRE) [
153]. GMP represents the gold standard in certifying the quality of pharmaceutical products and, thus, preventing the sale of substandard medicines, which can accelerate the emergence of resistant pathogens [
154].
This entry is adapted from the peer-reviewed paper 10.3390/applbiosci2020011