Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Human adenoviruses (HAdVs) are non-enveloped DNA viruses. Pathogenic human adenoviruses can infect various human cells, leading to respiratory, ocular, enteric, renal, and hepatic diseases.
- oncolytic adenovirus
- oncolysis
- immuno-oncology
1. Introduction
Based on WHO Global Health Estimates, the crude death rate per 100,000 population (CDR) decreased over the past 20 years. However, it was observed that the occurrence and mortality of malignant neoplasms increased in this period by almost 9%. Malignant neoplasms are now the second leading cause of death worldwide. Paradoxically, the most common cause of death (cardiovascular diseases) recorded a decrease in CDR [1]. Surgical excision of the tumor and chemo- and radiotherapy are the main methods of fighting cancer [2]. Chemo- and radiotherapy cause systematic toxicity, which is highly invasive for patients. Due to the non-specificity of those therapies, they also impact healthy cells [3]. Even though those therapies are effective in some cases, in others, the quality of a patient’s life is significantly reduced without any therapeutic effect [4].
Human adenovirus vectors (HAdVs) are promising candidates to overcome the drawback of current anticancer therapies. HAdVs are suitable vectors due to their high transgene capacity and flexibility in various modifications [5]. HAdVs have DNA genomes that are easily manipulated without attenuating viral replication and accommodate various transgenes [6]. HAdV clinical safety was evaluated and documented extensively; oncolytic HAdVs are the most frequently used oncolytic viruses in clinical trials, accounting for up to 42% of all trials [7]. Oncorine is the first oncolytic virus approved by the Chinese FDA in 2006 but not in Western countries. The majority of constructs based on human serotypes, such as ONCOS-102, LoAd703, TILT-123, ORCA-010, and CG0070, are under active clinical development [8]. The HAdV vectors are genetically modified to specifically respond to tumor-specific molecular alterations by replication and subsequent cell lysis [9]. This retargeting strategy reduced deleterious side effects and increased the therapeutic index of oncolytic viruses [9][10]. Moreover, HAdVs cause immunogenic cell death, engaging the immune cells to fight cancer [4].
2. Characterization of the Adenovirus Genome
Human adenoviruses (HAdVs) are non-enveloped DNA viruses. Pathogenic human adenoviruses can infect various human cells, leading to respiratory, ocular, enteric, renal, and hepatic diseases [11]. To date, 113 serotypes have been discovered (hadvwg.gmu.edu). Those serotypes are classified into seven “species”, A–G, formerly called subgroups [4]. Species differ by morphology, DNA homology, and oncogenicity [11][12]. Most species use coxsackievirus and adenovirus receptor (CAR) or CAR and silicic acid (SA) to infect cells [12]. However, species B and D utilize the co-stimulatory molecules CD80 and CD86 as cellular attachment receptors [13]. There are conflicting results [14][15][16][17][18][19] on the role of the membrane cofactor protein (MCP) CD46 in facilitating adenovirus 3 attachment to cells.
Adenoviruses contain a linear genome of 34–39 kb and a double-stranded DNA (Figure 1). Both DNA strands are flanked by two inverted terminal repeats (ITR) [20]. The HAdV genes are classified into early genes (E1A, E1B, E2, E3, E4) and late genes (L1–L5). All genes encode approximately 45 proteins [11][21][22]. Early genes are necessary for protein synthesis and replication of the viral genome. Late genes are responsible for virus escape from the cell and synthesis of the capsid structural proteins [20][23]. E1A is the first gene expressed after HAdV enters the host cell. Especially the E1A conserved region 2 (E1A-CR2) plays a critical role, as it interacts with retinoblastoma (Rb), stimulating the cells and viral DNA replication [21]. Two proteins encoded by the E1B gene are apoptosis inhibitor (19K) and inactivator of p53 (55K), respectively [10]. E1B inhibits apoptosis by blocking the function of the p53 tumor-suppressor protein. Adenoviruses without E1A and E1B cannot replicate in normal cells but replicate and cause cytopathic infections in the majority of tumor cells with lack of antioncogenes such as Rb and p53 [21][24]. The E2 gene encodes the DNA polymerases, the single-stranded DNA binding and terminal proteins [10][21]. The E3 gene provides defense mechanisms by inhibiting immune response [20]. Moreover, E3 encodes the adenoviral death protein. This protein induces cell lysis, making HAdV escape possible [10][21]. The last early E4 gene is involved in late viral gene expression and DNA replication. Proteins encoded by the E4 gene also limit the expression of cell proteins, promoting the expression of viral genes [10][11][21]. As previously mentioned, late genes are responsible for the synthesis of structural proteins. Late genes are placed under major late promoters (MLP): L2, L3, and L5 encode penton base, hexon, and fiber proteins, which are the main capsid structural segments. L1 encodes IIIa protein, which builds the inner side of the capsid. The L4 gene is responsible for the VIII protein synthesis, which combines the hexon components to improve capsid stability [10][20].
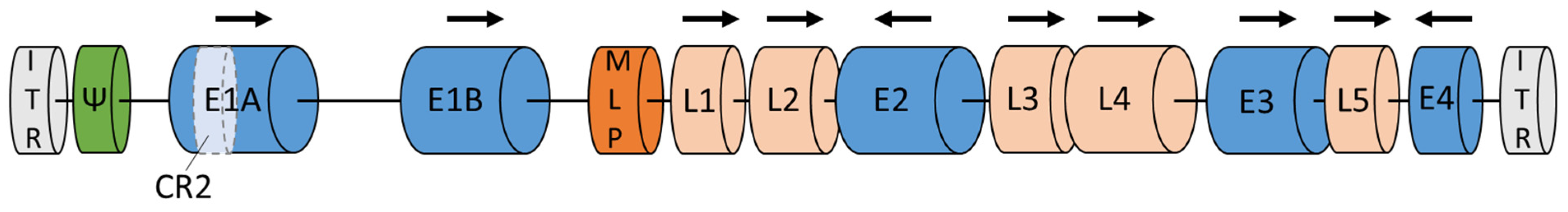
Figure 1. Adenoviral genome. Legend: E1–E4—early genes; L1–L5—late genes; ITR—inverted terminal repeat; MLP—major late promoter; Ψ—packaging sequences. Based on GenBank: MF502426.1; NCBI Reference Sequence: NC_001405.1 and [10].
The natural oncolytic activity of adenovirus can be improved by inserting transgenes into their native DNA [25]. Those transgenes are responsible for modifications in virus structure (capsid, fibers) or secretion of immune-activating peptides [2]. A proper understanding of the implications of various immune-affecting factors for cancer cells is crucial in developing modern anticancer viral therapies. As some of them can act as carcinogenic (TGF-B, IL-10, prostaglandin E) or anticancer (IL-2, IL-12, IL-15) agents, or both (GM-CSF, TNF), examination of new potential transgenes and their combination is necessary to obtain a universal, selective vector. Furthermore, the CD40 ligand (CD40L, CD154) induces apoptosis of tumor cells. It triggers several immune mechanisms, including a T-helper type 1 (T(H)1) response, which leads to the activation of cytotoxic T cells and reduction of immunosuppression [26]. Delolimogene mupadenorepvec (LOAd703) is an oncolytic adenovirus (serotype 5/35) that encodes for the transgenes CD40L and 4-1BBL, which activate both antigen-presenting cells and T cells. Moreover, LOAd703 infects cells via CD46, which enables B-cell infections [27]. Member of the tumor necrosis factor (TNF) superfamily OX40 ligand (OX40L) activates T cells [28]. On the other hand, vectors with immunostimulatory transgenes carry a risk of overdose. Immunostimulatory agents such as recombinant cytokines (INF-α, IL-2) used in the treatment of cancer have adverse consequences, including the acute phase response, cell and tissue abnormalities/injury, cytokine release/cytokine storm, tumor lysis syndrome, vascular leak, and autoimmunity [29]. The concentration of highly toxic cytokines could increase drastically if the vector replicates in patients’ cells with high efficiency. This could lead to serious systemic immune responses in patients [29]. When designing an oncolytic vector with the immunostimulatory transgene, it is crucial to consider the risks involved.
This entry is adapted from the peer-reviewed paper 10.3390/cancers15071947
References
- World Health Organization. Global Health Estimates 2019: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020.
- Jayalie, V.F.; Sekarutami, S.M. Combining Oncolytic Virus and Radiation Therapy for Cancer Management. J. Cancer Metastasis Treat 2022, 8, 17.
- Diaz Arguello, O.A.; Haisma, H.J. Apoptosis-Inducing TNF Superfamily Ligands for Cancer Therapy. Cancers 2021, 13, 1543.
- Bots, S.T.F.; Kemp, V.; Cramer, S.J.; van den Wollenberg, D.J.M.; Hornsveld, M.; Lamfers, M.L.M.; van der Pluijm, G.; Hoeben, R.C. Nonhuman Primate Adenoviruses of the Human Adenovirus B Species Are Potent and Broadly Acting Oncolytic Vector Candidates. Hum. Gene Ther. 2022, 33, 275–289.
- Hoare, J.; Campbell, N.; Carapuça, E. Oncolytic Virus Immunotherapies in Ovarian Cancer: Moving beyond Adenoviruses. Porto Biomed. J. 2018, 3, e7.
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.-C. Optimizing Oncolytic Virotherapy in Cancer Treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706.
- Yun, C.-O.; Hong, J.; Yoon, A.-R. Current Clinical Landscape of Oncolytic Viruses as Novel Cancer Immunotherapeutic and Recent Preclinical Advancements. Front. Immunol. 2022, 13, 953410.
- Boozari, B.; Mundt, B.; Woller, N.; Struver, N.; Gurlevik, E.; Schache, P.; Kloos, A.; Knocke, S.; Manns, M.P.; Wirth, T.C.; et al. Antitumoural Immunity by Virus-Mediated Immunogenic Apoptosis Inhibits Metastatic Growth of Hepatocellular Carcinoma. Gut 2010, 59, 1416–1426.
- Mathis, J.M.; Stoff-Khalili, M.A.; Curiel, D.T. Oncolytic Adenoviruses—Selective Retargeting to Tumor Cells. Oncogene 2005, 24, 7775–7791.
- Abudoureyimu, M.; Lai, Y.; Tian, C.; Wang, T.; Wang, R.; Chu, X. Oncolytic Adenovirus—A Nova for Gene-Targeted Oncolytic Viral Therapy in HCC. Front. Oncol. 2019, 9, 1182.
- Daussy, C.F.; Pied, N.; Wodrich, H. Understanding Post Entry Sorting of Adenovirus Capsids; A Chance to Change Vaccine Vector Properties. Viruses 2021, 13, 1221.
- Gao, J.; Zhang, W.; Ehrhardt, A. Expanding the Spectrum of Adenoviral Vectors for Cancer Therapy. Cancers 2020, 12, 1139.
- Short, J.J.; Vasu, C.; Holterman, M.J.; Curiel, D.T.; Pereboev, A. Members of Adenovirus Species B Utilize CD80 and CD86 as Cellular Attachment Receptors. Virus Res. 2006, 122, 144–153.
- Segerman, A.; Atkinson, J.P.; Marttila, M.; Dennerquist, V.; Wadell, G.; Arnberg, N. Adenovirus Type 11 Uses CD46 as a Cellular Receptor. J. Virol. 2003, 77, 9183–9191.
- Hall, K.; Blair Zajdel, M.E.; Blair, G.E. Defining the Role of CD46, CD80 and CD86 in Mediating Adenovirus Type 3 Fiber Interactions with Host Cells. Virology 2009, 392, 222–229.
- Gustafsson, D.J.; Segerman, A.; Lindman, K.; Mei, Y.-F.; Wadell, G. The Arg279Glu Substitution in the Adenovirus Type 11p (Ad11p) Fiber Knob Abolishes EDTA-Resistant Binding to A549 and CHO-CD46 Cells, Converting the Phenotype to That of Ad7p. J. Virol. 2006, 80, 1897–1905.
- Pache, L.; Venkataraman, S.; Reddy, V.S.; Nemerow, G.R. Structural Variations in Species B Adenovirus Fibers Impact CD46 Association. J. Virol. 2008, 82, 7923–7931.
- Wang, H.; Liaw, Y.-C.; Stone, D.; Kalyuzhniy, O.; Amiraslanov, I.; Tuve, S.; Verlinde, C.L.M.J.; Shayakhmetov, D.; Stehle, T.; Roffler, S.; et al. Identification of CD46 Binding Sites within the Adenovirus Serotype 35 Fiber Knob. J. Virol. 2007, 81, 12785–12792.
- Sirena, D.; Lilienfeld, B.; Eisenhut, M.; Kälin, S.; Boucke, K.; Beerli, R.R.; Vogt, L.; Ruedl, C.; Bachmann, M.F.; Greber, U.F.; et al. The Human Membrane Cofactor CD46 Is a Receptor for Species B Adenovirus Serotype 3. J. Virol. 2004, 78, 4454–4462.
- Singh, S.; Kumar, R.; Agrawal, B. Adenoviral Vector-Based Vaccines and Gene Therapies: Current Status and Future Prospects. In Adenoviruses; Desheva, Y., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-990-5.
- Wu, C.; Wei, F.; Xu, Z.; Wen, R.; Chen, J.; Wang, J.; Mao, L. Tropism and Transduction of Oncolytic Adenovirus Vectors in Prostate Cancer Therapy. Front. Biosci. (Landmark Ed.) 2021, 26, 866–872.
- Kuryk, L.; Møller, A.-S.; Vuolanto, A.; Pesonen, S.; Garofalo, M.; Cerullo, V.; Jaderberg, M. Optimization of Early Steps in Oncolytic Adenovirus ONCOS-401 Production in T-175 and HYPERFlasks. Int. J. Mol. Sci. 2019, 20, 621.
- Tseha, S.T. Role of Adenoviruses in Cancer Therapy. Front. Oncol. 2022, 12, 772659.
- Payne, S. Viruses, 1st ed.; Elsevier: Boston, MA, USA, 2017; ISBN 978-0-12-803109-4.
- Ranki, T.; Pesonen, S.; Hemminki, A.; Partanen, K.; Kairemo, K.; Alanko, T.; Lundin, J.; Linder, N.; Turkki, R.; Ristimäki, A.; et al. Phase I Study with ONCOS-102 for the Treatment of Solid Tumors—An Evaluation of Clinical Response and Exploratory Analyses of Immune Markers. J. Immunother. Cancer 2016, 4, 17.
- Diaconu, I.; Cerullo, V.; Hirvinen, M.L.M.; Escutenaire, S.; Ugolini, M.; Pesonen, S.K.; Bramante, S.; Parviainen, S.; Kanerva, A.; Loskog, A.S.I.; et al. Immune Response Is an Important Aspect of the Antitumor Effect Produced by a CD40L-Encoding Oncolytic Adenovirus. Cancer Res. 2012, 72, 2327–2338.
- Wenthe, J.; Naseri, S.; Labani-Motlagh, A.; Enblad, G.; Wikström, K.I.; Eriksson, E.; Loskog, A.; Lövgren, T. Boosting CAR T-Cell Responses in Lymphoma by Simultaneous Targeting of CD40/4-1BB Using Oncolytic Viral Gene Therapy. Cancer Immunol. Immunother. 2021, 70, 2851–2865.
- Rojas, J.M.; Alejo, A.; Avia, J.M.; Rodríguez-Martín, D.; Sánchez, C.; Alcamí, A.; Sevilla, N.; Martín, V. Activation of OX40 and CD27 Costimulatory Signalling in Sheep through Recombinant Ovine Ligands. Vaccines 2020, 8, 333.
- Ponce, R. Adverse Consequences of Immunostimulation. J. Immunotoxicol. 2008, 5, 33–41.
This entry is offline, you can click here to edit this entry!
