1. Nucleic Acid Microarrays
Nucleic acids (DNA and RNA) are the most important biomolecules found in all living cells and viruses with the primary function of storage and carrying genetic information [
138,
139]. Their chemical structure comprises of heterocyclic aromatic bases conjugated to a sugar–phosphate backbone, which is involved in a phosphodiester bond. Nucleic acid arrays, also known as DNA arrays, involve specific sequences of “probe” DNA molecules that are synthesized or deposited onto a solid flat surface [
20,
21,
22]. The application of these microarrays is to identify the target DNA sequence and to measure its concentration in a solution. In recent years, the applications of DNA have widened, allowing for gene expression analyses, transcription factor binding analyses, and genotyping. More specific details about DNA microarray fabrication can be referred to [
23,
45,
48,
140]. The articles in this section are reviewed based on their purpose of analysis, such as eukaryote and prokaryote, respiratory disease, and biomarker identification; the identification of gene mismatches and viruses; species authentication; and developing new strategies for DNA immobilization.
An 18S rRNA phylogenetic microarray [
135] detected eukaryotic organisms from marine sediments by targeting 18S rRNA operational taxonomical units (OTUs). The limitation of this approach was a decrease in the specificity with increasing sequence similarity. Using a DNA vertical flow paper microarray [
80],
neisseria meningitidis was detected with copies (38 and 2 × 10
6 per vertical flow assay) that were significantly similar to the Loop-Mediated Isothermal Amplification assay [
141].
Salmonella enterica was detected even at low levels (<10 colony forming units (CFUs)) in leafy greens using the DNA microarray-based PathogenDx system [
142]. A FRET based DNA microarray system [
104] specifically detected both 16S rDNA and 16S rRNA from
E. coli in 60 min.
The potential of the automated electronic microarray platform [
46] was explored to detect and differentiate multiple pathogens in a bovine respiratory disease complex and bovine enteric disease in a single sample. The microarray platform displayed a level of detection and differentiation of multiple pathogens similar to the multiplex PCR/RT-PCR approach. Six swine pathogens (PRDC, PCV2, PRRSV, Mhp, APP, HPS, CSFV, PPV, and SIV) were also simultaneously detected using an oligonucleotide microarray [
57].
A DNA microarray based on hairpin DNA molecules [
52] detected three protein biomarkers (carcinoembryonic antigen (CEA), α-fetoprotein (AFP), and carcinoma antigen 125 (CA125)) even at a low concentrations. These detection levels were either similar [
143,
144] or significantly higher [
145,
146] than those of earlier reported studies. Hypothermia-exposed murine lung samples were analyzed using the Agilent technologies DNA microarray platform (mouse, human, cDNA, oligo, number of genes) to identify the forensic biomarkers [
102]. The outcome of this study was the identification of 4094 genes (1699 upregulated and 2395 downregulated genes) that exhibited hypothermia-induced differential expression in the lungs. A circular RNA microarray-based [
101] functional experiment revealed cicRNA 7079 as a new antiapoptotic molecule in traumatic spinal cord injuries in mice. The differential DNA methylation profiles [
136] in Wharton’s jelly mesenchymal stem cells (WJ-MSCs), 5′Azacytidine treated WJ-MSC, and human cardiac tissue were studied using a customized 180 K human DNA methylation microarray. Catalytic hairpin assembly involves the use of a hairpin-shaped DNA, which, upon interaction with the target single-stranded analyte, leads to fluorescence signal amplification. An ultrasensitive fluorescence DNA microarray platform [
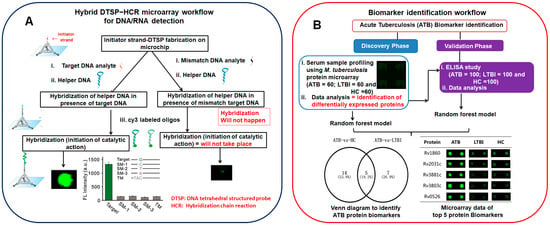
89] mediated by a tetrahedral DNA structured probe in combination with a hybridization chain reaction (HCR) was developed to detect both DNA and miRNA. The LOD of this method is 10 aM, and it distinguishes even a single-base mismatch in DNA (
Figure 1A). This approach [
58] was applied to a microarray platform to simultaneously detect multiple miRNAs in spiked human serum and the pathological cells Hela (cervical cancer cells) and MCF-7 (breast cancer cells) within 60 min. A silicon-based microarray chip (Si@Al@SiO
2 layered with a probe density of 1.9 ± 200 molecules μm
−2) [
92] was fabricated and validated by detecting melanocortin 1 receptor single-nucleotide polymorphisms.
A
BRCA1 gene DNA probe microarray containing silicon nanowires [
59] successfully discriminated the model mismatches in the sequence of
BRCA1. The synthetic DNA of seven antibiotic resistance genes from five cell lysates and three
E. coli strains were detected using an oligonucleotide microarray [
56]. The simultaneous detection of six avian influenza virus genes was achieved using an oligonucleotide microarray [
81]. A DNA microarray [
147] identified the serotypes of 14
E. coli isolates, and 6
E. coli isolates were found to be Shinga toxin-producing
E. coli. Of the 36 identified virulence genes,
hemL,
lpfA, and
iss were found to be more prevalent.
Hepatitis B virus (HBV) DNA was detected using the hybridization-induced silver nanoparticle (AgNP) aggregation strategy [
54]. This strategy involves the use of AgNPs conjugated with thiol-functionalized cy3-probe and hybrid nucleic acid probes (Tag-A and Tag-B). The presence of HBV DNA on the microarray only leads to AgNP aggregation and amplifies the fluorescence signal. In this case, a 1560-fold signal enhancement was achieved.
The ArrayTube2 DNA microarray [
55] with
cytb and 16S rDNA probes authenticated 10 different food fish specimens from a total of 67 fish species within 5 h. An oligonucleotide microarray [
148] was used to authenticate five marine mammal species (dolphins, seals, sea lions, white whales, and finless porpoises) in food and feed.
A multipolymer microarray spot platform [
77] with 16 different copolymers and various functional groups (amines, azides, or dibenzocyclooctynes) was explored for DNA probe immobilization. This DNA microarray platform resulted in improving the microarray sensitivity to be greater than that of the other existing immobilization approaches.
Figure 1. (
A) A pictorial workflow presentation of hybrid tetrahedral DNA structured probe in conjugation with hybridization chain reaction (DTSP-HCR) concept used to distinguish single-base mismatches in DNA, adapted from ([
89]) and (
B) an overview of acute tuberculosis (ATB) biomarker identification using a two-phase strategy (discovery phase and validation phase) adapted from ([
149]). Venn diagram and microarray chip visual analysis revealed the potential of 5 protein biomarkers to distinguish ATB and LTBI/HC. LTBI represents latent tuberculosis infection, and HC represents healthy control.
2. Protein Microarrays
Proteins are a class of biological macromolecules comprising of hundreds or thousands of amino acids covalently linked to each other via peptide bonds [
150]. Each protein is unique, with its own three-dimensional structure and amino acid sequence. In our body, they perform various useful functions (store and transport molecules, act as catalysts, transmit signals, provide strength to muscles and tissues, and protect us from infections). Proteins are immobilized on microarrays (protein microarrays) and explored to unravel proteome complexity and to identify protein functions [
26,
133,
151,
152]. They are classified into analytical, functional, and reverse-phase protein microarrays. However, an analytical protein microarray involves the use of antibodies. Functional protein microarrays are applied to study protein–protein interactions, enzyme–substrate relationships, biochemical activity, and immune responses. The fabrication of these microarrays is challenging due the requirement of a high amount of purified proteins. Hence, proteins are expressed in yeast and
E. coli expression systems. Reverse-phase protein microarrays (RPPAs) are high-throughput platforms that comprise of target proteins from cell or tissue lysates, sera/body fluids, or subcellular fractions immobilized as spots on the substrate [
153,
154,
155]. The subsequent step involves the use of specific tagged antibodies to detect the posttranslational modifications of proteins (e.g., phosphorylation) or the total protein expression levels. Considering the availability of the amine and carboxyl groups within the protein structure, the immobilization of proteins on a surface is relatively straight forward. Readers are referred to
Section 2.2 for protein immobilization chemistries. The articles in this section are presented based on their purpose of analysis, such as biomarker identification, mechanistic insights on infections, the study of antifouling properties, glycosylation profiling, the study of drug-binding proteins, vaccine candidate identification, target molecule detection, and the detection of SARS-CoV2 antigens in patient sera.
RPPAs [
36] were used to quantitatively screen protein biomarkers from hepatocellular carcinoma (HCC) patients’ sera. From the analysis, it was concluded that six proteins were identified with the potential to become diagnostic markers for HCC. A tuberculosis protein microarray [
149] was explored to screen the serum samples of active tuberculosis (ATB) patients, latent tuberculosis infection (LTBI) patients, and healthy controls (HCs). Four potential protein biomarkers were identified that can distinguish between ATB and LTBI, with a 93.3% sensitivity and 97.7% specificity (
Figure 1B).
The biological mechanism of the yang-deficiency constitution (intolerance to cold) at the protein level was studied using a protein microarray [
98]. The outcome was the identification of 85 differentially expressed plasma proteins (64 upregulated and 21 downregulated proteins), and it indicated that (low immune) metabolic disorders and endocrine disorders are the potential reasons. A protein microarray [
68] was evaluated to understand if it could provide more insights into pigs’ serological responses to
Mycoplasma hyopneumoniae infections. Compared to the commercial ELISA, the protein microarray proved to be an alternative and sensitive tool to detect
M. hyopneumoniae infections.
Diverse biomolecules (fibronectin, BSA, streptavidin, and RGD peptides) were immobilized on a functional hyperbranched PEG (polyethylene glycol) layer on silicon slides [
94] to achieve the antifouling property. BMSC and mouse L929 cells were successfully cultured on the microarray.
A lectin-based protein microarray [
78] was explored to obtain the glycosylation profile of the insulin-like growth factor receptors (IGF1 and IGF2) in colorectal carcinoma tissues. One of the important observations was that both receptors possessed high levels of α2,3 sialic acid residues; low levels of tri-/tetra-antennary complex type N-glycans with Gal β1,4 GlcNAc β1,6 Man; and high-mannose structures with terminal mannose. Similarly, a lectin-based protein microarray [
79] was used to analyze the glycosylation of recombinant dimeric IgA1 antibodies under the influence of different media supplements (asparagine, glutamine, and succinic acid). The absence of sugars (D-(+)-glucose, D-(+)-galactose, and D-(+)-mannose), use of one supplement, or replacement of one supplement with another resulted in glycan level fluctuations or changes in the glycan forms.
A
HuProt human protein microarray [
100], in combination with a bioinformatics analysis, was used to screen and identify potential doxorubicin (Dox; an anti-tumor drug)-binding protein targets. A total of 27 proteins were shortlisted, and studies on one of the proteins, HRAS (Harvey rat sarcoma viral oncogene homolog), revealed that Dox promotes HRAS-RAF complex formation.
A
Neisseria meningitidis antigen microarray [
85] was used in human phase I clinical trials to identify candidate vaccine proteins.
A macroporous polymer with a hydrophilic–hydrophobic property served as a layer for preparing protein arrays [
111]. Increased polymer layer hydrophilicity increased the analytical performance of the protein array. An acetylcholinesterase (AChE) antibody-coated chip was successfully applied for both AChE detection and the study of various enzyme kinetic assay parameters.
A SARS-CoV-2 variant protein microarray [
70] was used to profile the humoral immunity in a vaccinated (unvaccinated, partially vaccinated, and fully vaccinated) population. The major outcome was that full vaccination provided surrogate neutralization against all the mutants. The behavior of the immunoglobulins (IgG, IgA, and IgM isotypes) varied throughout the vaccination process. A coronavirus antigen microarray (COVAM) was fabricated using various recombinant proteins and antibodies [
156]. The COVAM selectively discriminated the viral (SARS-CoV-2 and influenza) infections with similar symptoms and showed a 77.2% sensitivity and 100% specificity. A flow-based chemiluminescence microarray immunoassay chip was developed using existing knowledge [
157,
158] for the identification of the SARS-CoV-2 IgG antibodies in human serum and plasma [
159]. Compared to the recomLine and recomWell systems, this microarray platform showed a 100% specificity and sensitivity within 8 min. In a protocol development study, a SARS-CoV-2 antigen-fabricated microarray was used to study the feasibility of assessing the interaction between the antigens and various immunoglobulins (IgG, IgM, and IgA) in patient sera [
160]. Another protocol study discussed the use of a SARS-CoV-2 antigen-fabricated microarray [
161] to profile protein sera samples. The study involved the preparation of proteins, microarray fabrication, sera profiling, and data analysis.
3. Peptide Microarrays
A peptide is a series of 2–50 amino acids covalently linked to each other via a dehydration reaction between the amino (-NH
2) moiety of amino acid A and the acid (-COOH) moiety of amino acid B. Peptides offer several advantages (ease of preparation, chemical stability, and compatibility with various immobilization strategies) over proteins [
32,
162]. Synthetic peptides are considered to be protein mimics and can be used for different biomedical applications [
163,
164,
165,
166]. Peptide-based array systems were first reported in the early 1990s [
12,
167], and, over the years, they have been used for studying enzyme functionality and inhibitor screening, the identification of disease biomarkers, and drug development. Peptide microarray synthesis can be performed in two ways [
32,
168]. The first approach is to perform in situ stepwise peptide synthesis on the array itself. The second approach is to spot or print the presynthesized peptides on the array slides via contact or noncontact mode. Here, the synthesis of peptides is performed via the widely known Merrifield solid-phase peptide synthesis technique. The second approach is mostly preferred over the first approach because its presynthesized peptide purity is excellent. The trend of preparing a peptide microarray via the second approach has persisted even in the articles published since 2018 [
51,
65,
66,
86,
87,
169,
170]. Readers are referred to
Section 2.2 for detailed peptide immobilization strategies. The articles in this section are presented based on their purpose of analysis, such as the identification of allergen-specific peptides, biomarker quantification, extracellular vesicle phenotype characterization, and the profiling of the SARS-CoV-2 antigen.
An allergome-wide 16-mer peptide microarray was constructed to detect allergen peptide-specific IgE, IgG4, and IgG [
51]. The outcome was the identification of allergen-specific humoral immunity.
A peptide microarray on a glass slide was designed to quantitatively screen the matrix metalloproteinase-2 secretion levels in normal cells (human colon epithelial cells) and four cancer cells (cervical, colorectal, hepatoma, and osteosarcoma cells) [
65]. The obtained microarray sensitivity was superior to the existing commercial kit. This was further improved by immobilizing peptides on a zinc oxide nanorod-polymer brush composite-grafted substrate [
66]. An antibody and peptide co-immobilized microarray was developed to study HEK derived extracellular vesicle (EV, membrane-bound vesicles) phenotype characterization [
87]. Compared to immobilized antibodies, the bradykinin-derived peptide displayed high binding to EVs.
A SARS-CoV-2 peptide microarray with peptides that were 15 amino acids long with a 5-amino-acid overlap was developed [
169]. The approach of peptide synthesis involved two steps, where the reference sequence of the SARS-CoV-2 genome, encoding 10 proteins, was identified and where a peptide library was prepared. The microarray was successfully applied for the epitope mapping of the IgM and IgG antibodies in COVID-19 infected patients’ sera. One of the identified peptide epitopes (FRKSN) could help in neutralizing RBD-ACE2 interacting antibodies. A peptide microarray against COVID-19 patients’ sera was constructed via the phage immunoprecipitation sequence (screened COVID-19 patients’ sera with nine human coronavirus genomes), ReScan (scanned patient serum antibodies and produced phage-displaying immunogenic antigens, i.e., peptides), paper microarray fabrication, and the identification of nine peptide candidates [
170]. The peptide microarray showed an 88% positive COVID-19 rate and a 75 and 100% sensitivity towards the sera of low- and high-neutralizing titers. A peptide sequence that recognizes the SARS-CoV-2 spike protein was designed, synthesized, and fabricated as a peptide microarray [
86]. This microarray was applied to the profiling of the linear epitopes of the spike protein in COVID-19 patients’ sera. A spike variant protein microarray was developed for the screening of drugs, neutralizing activity, and profiling humoral immunity using the sera of COVID-19 patients with varying levels of severity [
171]. Compared to severe patients, angiotensin-converting enzyme 2 (ACE2) inhibition was not strong in moderate or critical patients. The two ACE2 inhibitors (ramipril and perindopril) displayed dose-dependent inhibition in the case of all the spike variants, with the exception of B.1.617.3.
4. Glycan Microarrays
Glycans are a diverse chain of chemically linked monosaccharides present on the cell surfaces of all living organisms. These glycans are in conjugation with proteins (glycoproteins and proteoglycans) and lipids (glycosphingolipids). The interaction of glycans with glycan-binding proteins (GBP) helps in understanding the molecular mechanisms of various immunological events [
172,
173,
174]. The first reported glycan microarray in the year 2002 [
175,
176] studied GBP events using a small amount of a glycan sample [
28,
29,
177]. Later years witnessed the use of glycan microarrays in assessing glycan-processing enzyme characterization, the discovery of functional glycans, drug discovery, studying weak carbohydrate interactions, and pathogen diagnosis [
76,
178,
179,
180]. The immobilization of glycans on a solid surface is generally performed after the target glycan is modified with a reactive functional group (NHS, amines, alkenes, carbonyls, and thiols). The articles presented in this section are based on their purpose of analysis, such as glycan quantification; carbohydrate–protein (immunoglobulins, lectins, and the spike protein of SARS-CoV-2) interaction studies; the identification of viruses, foodborne bacteria, and enzyme activity; and IC50 value estimation.
Different strategies for glycan immobilization on amine functional silicon oxide and glass surfaces were studied [
72], and the glycan quantity was quantified using a model lectin Concanvalin A. The glycan chip has a shelf-life activity of more than 5 months.
A glycan microarray [
106] (from the Consortium for Functional Glycomics with 610 natural and synthetic glycans) was used to identify the secondary-binding site in human macrophage galactose-type lectin. Two different oligosaccharides ((
1) Glc-(α-1,2)-Glc and (
2) Man-(α-1,2)-Man-(α-1,2)-Man: central mannose with β-1,4) fabricated on three commercially available slides were used to study the weak-affinity interactions between carbohydrates and lectins [
76]. Glycan microarrays with N-glycolylneuraminic acid (Neu5Gc) and N-Acetylneuraminic acid (Neu5Ac) glycans revealed that different intravenous immunoglobulin (IVIG) preparations show that IgA has cross-reactivity against several Neu5Ac-glycans and that anti-Neu5Gc has high specific IgG reactivity [
63]. Carbohydrate microarrays with varying densities (mono-, bi-, and tetravalent dendrons) were fabricated via a spot-wise strain-promoted azide–alkyne cycloaddition approach and were evaluated for carbohydrate–lectin interactions [
103]. With the increasing valency of the dendron structure from mono- to tetravalent, the binding affinity of the lectins (Pisum sativum, Wisteria floribunda, the extracellular domains of the human lectin receptors DC-SIGN, DC-SIGNR, Langerin, and Dectin-2) also increased. A carbohydrate microarray fabricated on a polymethylacrylic acid glass substrate [
75] was studied for its affinity to the SARS-CoV-2 spike protein. The two carbohydrates heparin and fucoidan specifically recognized the spike protein of SARS-CoV-2. A glycan microarray consisting of 800 components identified 26 mAbs from a pool of 516 human mAbs [
64]. The further analysis of these 26 mAbs provided insights on their cellular origins and binding specificities. This could help in designing future diagnostic kits and in the therapeutic application of various diseases.
A carbohydrate microarray [
74] fabricated on CC-polyHEMA-slides had a high carbohydrate-loading ability and a low LOD of 9.28–928 µM as compared to the existing arrays. The two carbohydrates sialic acid and ManA2 interacted with the influenza A virus H1N1-hemagglutinin (an IAV envelope glycoprotein).
Various mannose-coated microarrays were constructed [
61] to identify foodborne bacteria. A mannose array chip prepared on a thiol-functionalized glass displayed a better detection performance of the target bacteria over NHS functionalized glass slides. The mannose array chip showed the successful positive binding of 8 out of 12
salmonella isolates and 7 out of 9 diarrheagenic
E. coli isolates with varying binding affinities. A total of 31 neutral N-glycans from ovalbumin were isolated and immobilized on a nitrocellulose-coated glass slide to develop a glycan array chip [
73]. A glycan array chip with 6
Helicobacter pylori (
H. pylori) lipopolysaccharides (LPSs) and 26,695 strains was constructed on a nitrocellulose-coated glass slide [
84]. This glycan chip successfully detected the specific anti-
H. pylori LPS IgG response and distinguished it from noninfected human sera. Hybrid-type N-glycans with bisecting GlcNAc showed a specific affinity to wheat-germ agglutinin, and complex-type N-glycans with bisecting GlcNAc displayed a low binding affinity to WGA.
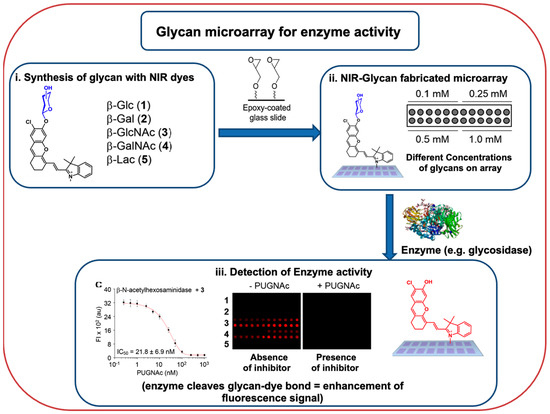
Glycosidase enzyme (β-glucosidase from almonds, β-galactosidase from A. oryzae, and β-N-acetylhexosaminidase from S. pneumoniae) activities were studied using a carbohydrate microarray (
Figure 2) comprised of a series of different glycosylated near-infrared probes (five probes and five carbohydrates) [
62]. These arrays were also explored to estimate the IC
50 values of glycosidase enzyme inhibitors (CBE, DGJ, and PUGNAc).
Figure 2. A generic workflow of the application of glycan microarray chip to efficiently profile enzyme activities and determine enzyme inhibitor IC
50 values; adapted from ([
62]).
5. Antibody Microarrays
Since the COVID-19 pandemic started, the word “antibodies” has become a much more familiar name in every household around the globe. Antibodies are Y-shaped protein molecules with a specific binding site for antigens. They are produced through hybridoma technology, which was introduced in 1975 by Kohler and Milstein [
181]. This has resulted in the production of several monoclonal antibodies for therapeutic and diagnostic applications [
182,
183,
184,
185,
186]. Antibodies and their derivatives (single-chain variable fragments, antigen-binding fragments, and nanobodies) are printed on microarrays to study the signaling pathways of various disease states, protein–protein interactions, and drug mechanisms and to identify the protein markers of autoimmune diseases, infectious diseases, and neurodegenerative diseases [
34]. The performance of these antibody microarrays is significantly comparable to the conventional ELISA and Western blotting methods in terms of high throughput, multiplexing, sensitivity, and sample diversity. The articles presented in this section are based on their purpose of analysis, such as polysaccharide profiling, biomarker identification, target protein (interleukins (ILs), TNF-α, andSARS-CoV-2 antigens) and extracellular vesicle capture, and virus screening.
The comprehensive microarray polymer profiling approach uses a series of carbohydrate-binding modules and monoclonal antibodies to detect glycan epitopes. This strategy [
60] is used to track polysaccharides during the winemaking process.
A novel biomarker CCL5 (out of 274 detected human proteins) from primary colorectal cancer tissue was identified using an antibody microarray [
97]. Coronary artery stenosis serum protein profiling was studied using an antibody microarray [
95] to identify the novel biomarkers associated with the disease. Irrespective of gender, the levels of cadherin-P, IL-5, glutathione S-transferase Mu, and neuronal nitric oxide synthase increased significantly. Additionally, the levels of nine proteins increased 4–30 fold, specifically in men only. The protein list included fibroblast growth factor 1, collagen alpha-1(II) chain, granulocyte–macrophage colony-stimulating factor, IL-1α, angiopoietin-2, granulocyte colony-stimulating factor, lymphocyte cell-specific protein tyrosine kinase, and kappaB kinase b. Similarly, in the case of women only, the levels of eight proteins decreased 4–15 fold. The protein list included cyclin-dependent kinase 1, DNA fragmentation factor subunit alpha, early E1A protein, calponin, ADP-ribosylation factor 6, alpha skeletal muscle actin, thyroid hormone receptor alpha, and alpha-methylacyl-CoA racemase. Using the
E. coli proteome chip microarray [
69], IgM and IgG antibodies were screened from 80 schizophrenia patient samples and 40 healthy individuals. They identified three potential antibody biomarkers (yjjU, livG, and ftsE) that could help in differentiating adult-onset schizophrenia and healthy individuals.
PDA microarray spots on a layer of amorphous fluoropolymer-CYTOP-glass slides [
50] were used for protein (IgG as model molecule) immobilization and binding studies. Under optimized binding conditions, an anti-IL-6 protein microarray showed specificity to IL-6 in the presence of spiked IL-10. Interestingly, in one study, the explored aptamers were used [
99] to immobilize antibodies and to create antibody microarrays. The applicability of this chip was demonstrated by immobilizing adalimumab, a TNF-α binding antibody. The antibody specifically bound to TNF-α and not to other proteins (IL-5, CD2, PD-L1, and TGF-
β3). In comparison to conventional antibody microarrays, the antibody microarray developed via the DDI approach [
83] efficiently captured the extracellular vesicles of the HEK-293 cells from the diluted solutions.
Six upper respiratory tract viral pathogens (IAV, IBV, RSV, hAdV, hPIV2, and hPIV3) were simultaneously screened using an antibody microarray [
82]. The advantage of this microarray over the ELISA is the requirement of low reagent volumes and the multiplexing of six monoclonal antibodies on a single chip.
A MosaiQ COVID-19 antibody microarray [
134] was used to detect antibodies against the SARS-CoV2 antigens in French patients’ samples (whole blood, plasma, or serum specimens). The overall sensitivity (88%) of the MosaiQ test was significantly higher than that of the other existing commercial competitors (< 79%). This microarray proved to be a high-throughput assay with a very high sensitivity and specificity, greater than those of the ELISA. The VaxArray Coronavirus SeroAssay kit, containing nine coronavirus spike proteins [
96], was used to perform the simultaneous analysis of the antibody responses to all of the nine printed antigens in 2 h. This platform displayed a 0.32–1.99 ng/mL limit of quantification and a 76–911-fold linear dynamic range. A disposable “pre-equilibrium digital enzyme linked immunoassay (PEDELISA)” microarray platform was developed to monitor various cytokine (IL-6, TNF-α, IL-1β, and IL-10) levels in severely ill COVID-19 patients within 4 h [
187]. The advantage of the PEDELISA over the other existing systems is its high sensitivity and interassay repeatability. Both the IL-6 and IL-10 levels in the patients receiving tocilizumab (IL-6 inhibitor) were heterogeneous and indicated the need for a personalized strategy.
A proteomic antibody microarray [
188] was used to investigate the differentially expressed proteins (DEPs) in the human sera of people administered with three different traditional Chinese medicinal constitutions. For the phlegm-dampness constitution, the proteins were upregulated, and, in case of the phlegm-damp-heat constitution, the proteins were downregulated. This could be due to hyperthyroidism or a low immunity.
6. Aptamer Microarrays
Aptamers are small single-stranded DNA or RNA oligonucleotides that possess the special ability, similar to antibodies, of binding to the target molecules [
189]. Since they are synthesized from nucleic acids and have an affinity similar to antibodies, they are known as chemical antibodies as well. The typical length of an aptamer is in the range of 20–60 or 80 nucleotides. The high affinities and specificities displayed by these aptamers to the target molecules, either a complex large protein or a simple small inorganic molecule, is similar to that of antibodies [
190]. Hence, they are considered to be an efficient alternative to antibodies. Additionally, compared to antibodies, aptamer production is simple and cost-effective and has a high batch-to-batch reproducibility and stability and a very low immunogenicity [
191,
192]. Due to this added feature of having a very low immunogenicity, they are also considered to be potential agents for therapeutic and diagnostic applications [
192,
193]. Researchers also explore them in microarrays and study them with protein quantification [
193]. Aptamers are synthesized through a process called the systematic evolution of ligands through exponential enrichment. For detailed information, readers are referred to [
194,
195,
196]. The fabrication of aptamer microarrays is performed via two methods (the in situ synthesis method and the postsynthesis method). The in situ synthesis method involves the direct synthesis of aptamers on the microarray surface. In case of the postsynthesis method, the synthesized aptamers are spotted on functionalized (carboxyl, hydroxyl, and amine) surfaces via noncontact or contact mode. Considering that aptamers are alternatives to antibodies, it is interesting to note that they were also used as an alternative linker molecule to immobilize the antibodies on a microarray platform [
99].
Microfungi are known to produce toxic secondary metabolites, known as mycotoxins, which are harmful to both humans and animals [
197,
198]. There are around 300 mycotoxins of which 6 (aflatoxins, trichothecenes, zearalenone, fumonisins, ochratoxins, and patulin) are majorly found in various foods, such as maize, wheat, rice, cereals, grapes, apples, etc. According to the guidelines from various public health and government bodies in the USA and the European Union, the allowed level of some of these toxins is as follows: aflatoxins (B1, B2, G1, and G2) are allowed at 20 µg/Kg in the USA and at 4–15 µg/Kg in total (2–12 µg/Kg for B1 alone), ochratoxin A is allowed at 2–10 µg/Kg in the EU (not set in the USA), fumonisins (B1, B2, and B3) are allowed at 200–1000 µg/Kg in the EU and at 2000–4000 µg/Kg in the USA, patulin is allowed at 50 µg/Kg in the EU and at 10–50 µg/Kg in the USA, and zearalenone is allowed at 20–100 µg/Kg in the EU (not set in the USA). Multiple mycotoxins (ochratoxin A, aflatoxin B1, and fumonisin B1) in spiked wheat, corn, and rice samples were simultaneously detected using the FRET approach [
71]. With varying mycotoxins, the LOD of this system ranged from 0.21 to 15.4 pg/mL.
Organophosphorus pesticides are mainly used to protect crops from pests [
199]. Over the years, their extensive use has caused more harm to human health [
200]. According to the EU, the established maximum residue values of two of the pesticides, namely, phoxim and parathion, and isocarbophos in fresh vegetables and fruits are 50 µg/kg, 10 µg/kg, and 50 µg/kg, respectively [
201]. A high-throughput fluorescence-based aptamer microarray was developed for the detection of multiple organophosphorus pesticides in food [
202]. Thioflavin T was used as the fluorescence indicator, which was bound to the aptamer. The interaction of the target organophosphorus pesticides (phoxim, parathion, fensulfothion, and isocarbophos) with the aptamer resulted in the displacement of Thioflavin T and a decrease in the fluorescence intensity. The LOD of this microarray was 25.4 ng/mL for phoxim, 12.0 ng/mL for parathion, 7.7 ng/mL for fensulfothion, and 9.9 ng/mL for isocarbophos, respectively.
This entry is adapted from the peer-reviewed paper 10.3390/biom13040602