Oxidative stress in chickens caused by dietary, environmental, and pathological variables influences how well chickens perform as well as the quality of meat and eggs. Lead (Pb) and cadmium (Cd) are two examples of heavy metals that are harmful for chicken health. They can cause oxidative stress by increasing the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and blocking antioxidants from protecting cells from increased amounts of free radicals. The oxidative state of heavy metals, their interactions with endogenous antioxidants, and chemical processes all affect how hazardous they are to the body.
- heavy metals
- phytogenic compounds
- oxidative stress
- poultry
1. Introduction
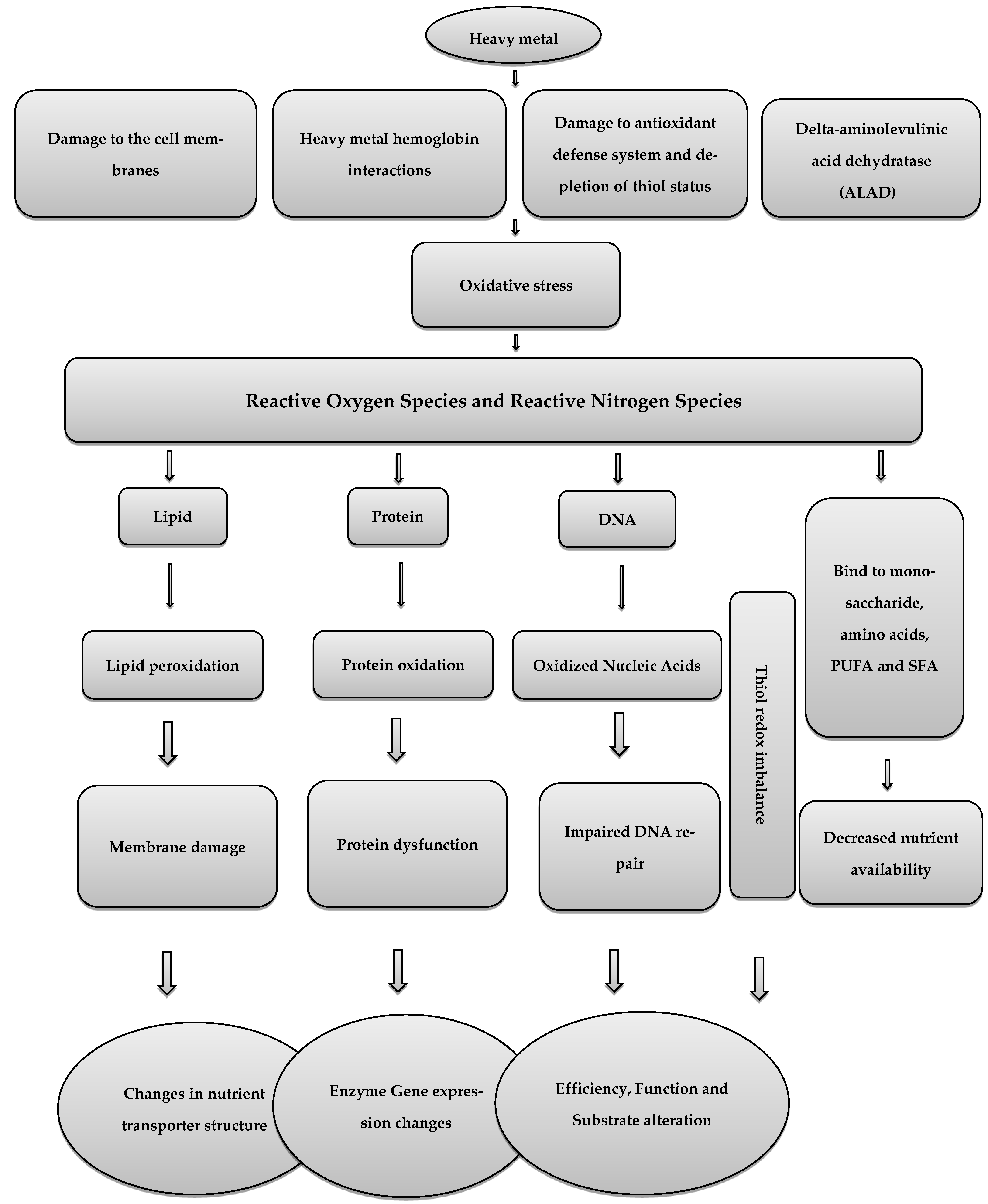
2. Heavy Metals and Oxidative Stress in Poultry
2.1. Pb
2.2. Cd and As
2.3. Mitigation of Oxidative Stress in Poultry
This entry is adapted from the peer-reviewed paper 10.3390/poultry2020019
References
- Bacou, E.; Walk, C.; Rider, S.; Litta, G.; Perez-Calvo, E. Dietary oxidative distress: A review of nutritional challenges as models for poultry, swine and fish. Antioxidants 2021, 10, 525.
- Shakeri, M.; Oskoueian, E.; Le, H.H.; Shakeri, M. Strategies to combat heat stress in broiler chickens: Unveiling the roles of selenium, vitamin E and vitamin C. Vet. Sci. 2020, 7, 71.
- Shakeri, M.; Le, H.H. Deleterious Effects of Heat Stress on Poultry Production: Unveiling the Benefits of Betaine and Polyphenols. Poultry 2022, 1, 147–156.
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60.
- Shakeri, M.; Cottrell, J.J.; Wilkinson, S.; Zhao, W.; Le, H.H.; McQuade, R.; Furness, J.B.; Dunshea, F.R. Dietary betaine improves intestinal barrier function and ameliorates the impact of heat stress in multiple vital organs as measured by evans blue dye in broiler chickens. Animals 2019, 10, 38.
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313.
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972.
- Khafaga, A.F.; El-Hack, A.; Mohamed, E.; Taha, A.E.; Elnesr, S.S.; Alagawany, M. The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: A review. Environ. Sci. pollut. Res. 2019, 26, 4588–4604.
- Nai, G.A.; Marin, F.F.; Queiroz, L.M.M.; Estrella, M.P.S. Respiratory tract cadmium-induced injuries—poisoning via intake and water pH could influence their genesis? An experimental study in rats. Comp. Clin. Path. 2017, 26, 997–1002.
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267.
- Papanikolaou, N.C.; Hatzidaki, E.G.; Belivanis, S.; Tzanakakis, G.N.; Tsatsakis, A.M. Lead toxicity update. A brief review. Med. Sci. Monit. 2005, 11, RA329.
- Korish, M.A.; Attia, Y.A. Evaluation of heavy metal content in feed, litter, meat, meat products, liver, and table eggs of chickens. Animals 2020, 10, 727.
- Baloš, M.Ž.; Jakšić, S.; Pelić, D.L. The role, importance and toxicity of arsenic in poultry nutrition. Worlds Poult. Sci. J. 2019, 75, 375–386.
- Zheng, S.; Wang, Q.; Yuan, Y.; Sun, W. Human health risk assessment of heavy metals in soil and food crops in the Pearl River Delta urban agglomeration of China. Food Chem. 2020, 316, 126213.
- Okoye, P.; Ajiwe, V.; Okeke, O.; Ujah, I.; Asalu, U.; Okeke, D. Estimation of heavy metal levels in the muscle, gizzard, liver and kidney of broiler, layer and local (cockerel) chickens raised within Awka metropolis and its environs, Anambra state, south eastern Nigeria. J. Environ. Prot. 2015, 6, 609.
- Bakalli, R.; Pesti, G.; Ragland, W. The magnitude of lead toxicity in broiler chickens. Vet. Hum. Toxicol. 1995, 37, 15–19.
- Dobrzański, Z.; Gorecki, H.; Chojnacka, K.; Gorecka, H.; Synowiec, M. Effect of dietary humic preparations on the content of trace elements in hens’ eggs. Am. J. Agric. Biol. Sci. 2007, 2, 234–240.
- Abduljaleel, S.A.; Shuhaimi-Othman, M. Metals concentrations in eggs of domestic avian and estimation of health risk from eggs consumption. J. Biol. Sci. 2011, 11, 448–453.
- Hunton, P. Research on eggshell structure and quality: An historical overview. Braz. J. Poult. Sci. 2005, 7, 67–71.
- Dauwe, T.; Janssens, E.; Bervoets, L.; Blust, R.; Eens, M. Heavy-metal concentrations in female laying great tits (Parus major) and their clutches. Arch. Environ. Contam. Toxicol. 2005, 49, 249–256.
- Ebrahimi, R.; Faseleh Jahromi, M.; Liang, J.B.; Soleimani Farjam, A.; Shokryazdan, P.; Idrus, Z. Effect of dietary lead on intestinal nutrient transporters mRNA expression in broiler chickens. BioMed Res. Int. 2015, 2015, 149745.
- Flora, S.; Mittal, M.; Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 2008, 128, 501.
- Ye, F.; Li, X.; Li, F.; Li, J.; Chang, W.; Yuan, J.; Chen, J. Cyclosporin A protects against Lead neurotoxicity through inhibiting mitochondrial permeability transition pore opening in nerve cells. Neurotoxicology 2016, 57, 203–213.
- Ma, L.; Liu, J.-Y.; Dong, J.-X.; Xiao, Q.; Zhao, J.; Jiang, F.-L. Toxicity of Pb2+ on rat liver mitochondria induced by oxidative stress and mitochondrial permeability transition. Toxicol. Res. 2017, 6, 822–830.
- Gurer-Orhan, H.; Sabır, H.U.; Özgüneş, H. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 2004, 195, 147–154.
- Wang, J.; Zhu, H.; Yang, Z.; Liu, Z. Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J. Pharmacol. 2013, 45, 395.
- Liu, C.-M.; Ma, J.-Q.; Sun, Y.-Z. Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2012, 258, 330–342.
- Neal, A.P.; Guilarte, T.R. Molecular neurobiology of lead (Pb2+): Effects on synaptic function. Mol. Neurobiol. 2010, 42, 151–160.
- Zhu, M.; Li, H.; Bai, L.; Wang, L.; Zou, X. Histological changes, lipid metabolism, and oxidative and endoplasmic reticulum stress in the liver of laying hens exposed to cadmium concentrations. Poult. Sci. 2020, 99, 3215–3228.
- Branca, J.J.V.; Morucci, G.; Pacini, A. Cadmium-induced neurotoxicity: Still much ado. Neural Regen. Res. 2018, 13, 1879.
- Li, Y.-x.; Xiong, X.; Chun-ye, L.; Feng-song, Z.; Wei, L.; Wei, H. Cadmium in animal production and its potential hazard on Beijing and Fuxin farmlands. J. Hazard. Mater. 2010, 177, 475–480.
- Fan, R.; Hu, P.-c.; Wang, Y.; Lin, H.-y.; Su, K.; Feng, X.-s.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66.
- Akyolcu, M.; Ozcelik, D.; Dursun, S.; Toplan, S.; Kahraman, R. Accumulation of cadmium in tissue and its effect on live performance. In Proceedings of the Journal de Physique IV (Proceedings), Porto, Portugal, 8–10 September 2003; pp. 33–36.
- Stohs, S.J.; Bagchi, D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336.
- Patrick, L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern. Med. Rev. 2003, 8, 106–128.
- Castagnetto, J.M.; Hennessy, S.W.; Roberts, V.A.; Getzoff, E.D.; Tainer, J.A.; Pique, M.E. MDB: The metalloprotein database and browser at the Scripps Research Institute. Nucleic Acids Res. 2002, 30, 379–382.
- Kern, M.; Wisniewski, M.; Cabell, L.; Audesirk, G. Inorganic lead and calcium interact positively in activation of calmodulin. Neurotoxicology 2000, 21, 353–363.
- Sun, X.; Tian, X.; Tomsig, J.L.; Suszkiw, J.B. Analysis of differential effects of Pb2+ on protein kinase C isozymes. Toxicol. Appl. Pharmacol. 1999, 156, 40–45.
- Marchetti, C. Role of calcium channels in heavy metal toxicity. Int. Sch. Res. Not. 2013, 2013, 184360.
- Gu, J.; Dai, S.; Liu, Y.; Liu, H.; Zhang, Y.; Ji, X.; Yu, F.; Zhou, Y.; Chen, L.; Tse, W.K.F. Activation of Ca2+-sensing receptor as a protective pathway to reduce Cadmium-induced cytotoxicity in renal proximal tubular cells. Sci. Rep. 2018, 8, 1–13.
- Sarkar, A.; Ravindran, G.; Krishnamurthy, V. A brief review on the effect of cadmium toxicity: From cellular to organ level. Int. J. Biotechnol. Res. 2013, 3, 17–36.
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214.
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281.
- Zhao, H.; He, Y.; Li, S.; Sun, X.; Wang, Y.; Shao, Y.; Hou, Z.; Xing, M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor dependent pathways in chicken immune organs. Oncotarget 2017, 8, 40327.
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107.
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Kidd, M.T. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants 2019, 8, 235.
- Estévez, M. Oxidative damage to poultry: From farm to fork. Poult. Sci. 2015, 94, 1368–1378.
- Lauridsen, C. From oxidative stress to inflammation: Redox balance and immune system. Poult. Sci. 2019, 98, 4240–4246.
- McGill, M.R.; Du, K.; Weemhoff, J.L.; Jaeschke, H. Critical review of resveratrol in xenobiotic-induced hepatotoxicity. Food Chem. Toxicol. 2015, 86, 309–318.
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807.
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. Toxicol. Sci. 2002, 69, 149–156.
- Winiarska-Mieczan, A. Protective effect of tea against lead and cadmium-induced oxidative stress—A review. Biometals 2018, 31, 909–926.
- Mężyńska, M.; Brzóska, M.M.; Rogalska, J.; Piłat-Marcinkiewicz, B. Extract from Aronia melanocarpa L. berries prevents cadmium-induced oxidative stress in the liver: A study in a rat model of low-level and moderate lifetime human exposure to this toxic metal. Nutrients 2018, 11, 21.
- Fang, Y.; Zhong, R.; Chen, L.; Feng, C.; Sun, H.; Zhou, D. Effects of astaxanthin supplementation on the sperm quality and antioxidant capacity of ram semen during liquid storage. Small Rumin. Res. 2015, 130, 178–182.
- Najafi, D.; Taheri, R.A.; Najafi, A.; Shamsollahi, M.; Alvarez-Rodriguez, M. Effect of astaxanthin nanoparticles in protecting the post-thawing quality of rooster sperm challenged by cadmium administration. Poult. Sci. 2020, 99, 1678–1686.
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146.
- Rubiolo, J.; Vega, F. Resveratrol protects primary rat hepatocytes against necrosis induced by reactive oxygen species. Biomed. Pharmacother. 2008, 62, 606–612.
- Alagawany, M.; El-Hack, A.; Mohamed, E.; El-Kholy, M.S. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ. Sci. pollut. Res. 2016, 23, 6774–6782.
- Chakravarthi, S.; Jessop, C.E.; Bulleid, N.J. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 2006, 7, 271–275.
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Chen, X.; Geng, Z.; Hu, H.; Zhang, C. Effects of resveratrol on growth performance, intestinal development, and antioxidant status of broilers under heat stress. Animals 2021, 11, 1427.
- Olas, B.; Wachowicz, B.; Stochmal, A.; Oleszek, W. Inhibition of oxidative stress in blood platelets by different phenolics from Yucca schidigera Roezl. bark. Nutrition 2003, 19, 633–640.
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Syimal’ain Azmi, N.; Noor Mohamad Zin, N.S.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement. Altern. Med. 2017, 17, 1–17.
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233.
- Bhattacharya, S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm. Biol. 2017, 55, 349–354.
- Mehrandish, R.; Rahimian, A.; Shahriary, A. Heavy metals detoxification: A review of herbal compounds for chelation therapy in heavy metals toxicity. J. Herbmed Pharmacol. 2019, 8, 69–77.
- Das, B.; Chaudhuri, K. Amelioration of sodium arsenite induced toxicity by diallyl disulfide, a bioactive component of garlic: The involvement of antioxidants and the chelate effect. RSC Adv. 2014, 4, 20964–20973.
- Adegboyega, A.; Odunola, O. The modulatory effects of aqueous extracts of Viscum album and garlic on sodium arsenite induced toxicity in Wistar albino rat. J. Chem. Pharm. Res. 2012, 4, 4698–4701.
- Sharmila Banu, G.; Kumar, G.; Murugesan, A. Effect of ethanolic leaf extract of Trianthema portulacastrum L. on aflatoxin induced hepatic damage in rats. Indian J. Clin. Biochem. 2009, 24, 414–418.
- Bjørklund, G.; Rahaman, M.S.; Shanaida, M.; Lysiuk, R.; Oliynyk, P.; Lenchyk, L.; Chirumbolo, S.; Chasapis, C.T.; Peana, M. Natural dietary compounds in the treatment of arsenic toxicity. Molecules 2022, 27, 4871.
