Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Titanium and its alloys have been extensively used as implant materials in clinic settings. However, implant-associated bacterial infection or inflammation remains a primary cause of implantation failure, which threatens human health, and has already become a global issue. A superhydrophobic surface endowed with a water contact angle higher than 150° has attracted widespread attention in antibacterial applications for their self-cleaning and low-adhesion properties, which has emerged as an important path in preventing biofilm formation.
- superhydrophobic
- titanium
- implant
- antibacterial
1. Introduction
It is well known that titanium-based alloys, as the gold standard, are utilized in applying repair to a bone or a specific part replacement, such as dental implants, bone plates, and screws, due to their high strength, good anticorrosion performance, and sufficient biocompatibility [1]. Typically, ASTM grade V titanium (Ti6Al4V) is one of the applied materials, which is most frequently used in orthopedic applications. The mechanical characteristics and chemical reactivity of titanium alloys of grades II, III, and IV are significantly different to those of grade V, making them more appropriate to be utilized in dental implant applications [2]. As a kind of biophilic metal, titanium is highly biocompatible with bone, which is reliant on surface characteristics, such as surface roughness [3], chemistry [4], and wettability. In comparison to smoother surfaces, surface topographical changes at the micrometer level, such as those caused by acid etching and sandblasting, have a remarkable impact on cell differentiation, local factor creation, bone formation, and (ultimately) osseointegration [5][6]. The surface roughness, chemistry, and wettability can be controlled to support osseointegration properties [7]. For example, the titanium substrates of nanoscale structures can enhance osteoblast differentiation and local factor creation when combined with micro-/submicro-scale roughness, suggesting the possibility of better implant osseointegration abilities [8]. However, infection may occur after medical device implantation due to bacterial adhesion in a certain period [9][10].
Therefore, more and more researchers are interested in strategies used to prevent bacterial growth and biofilm formation, relying on either the chemical approaches of bactericidal activity to kill the bacteria attached to the surface or the physical approaches of antibiofouling activity to inhibit the initial bacterial attachment to the surface [11]. A wide range of antimicrobial agents, including antibiotics, bacteriostatic and bactericidal chemicals (such as chlorhexidine, triclosan, silver preparations, and antimicrobial peptides) [12], fluoride, and plant extracts, inhibiting metabolic enzymes and their small molecular substances [13][14][15], have been used to prevent biofilm maturation by bactericidal inhibition, the inhibition of bacterial adhesion, and the destruction of the extracellular matrix of plaque. However, such methods do not have the value of long-term use and may carry certain risks, such as toxicity and drug resistance. For the bactericidal method, the layer of bacteria killed on the surface may have an inhibitory effect on the further bacterial killing in a deeper surface, and the remaining bacterial film could be conducive to the attachment of live bacteria [16]. It is highly desirable to prevent biofilm formation using antimicrobials, rather than biocidal agents. Consequently, it is generally considered that approaches to prevent the initial bacterial attachment are much better than those aiming to kill the attached bacteria. Thus, more research should focus on the inhibition of the initial bacterial adhesion on the surface of the implants.
Over the past several years, superhydrophobic surfaces endowed with a water contact angle more than 150° have attracted great attention because of their unique properties, such as water repellency [17][18], self-cleaning [19][20][21], anticorrosion [22][23][24][25], anti-icing [26][27], and oil–water separation [28][29]. It was not until 1997 that Barthlott and Neinhuis concluded that the self-cleaning effect of the lotus leaf (called the lotus effect) is attributed to the presence of the papilla on the microstructure and epicuticular wax [30]. Based on this principle, Jiang et al. revealed that the primary cause generating the superhydrophobicity of lotus leaves was due to the synergistic effect of hierarchical micro/nanoscale structures and low-surface energy material modification [31], which can form an air layer on the surface and physically inhibit bacterial adhesion. From that point, research on wettability of solid surfaces was renewed as a dynamic research topic [32]. It was reported that superhydrophobic surfaces have minimal bacterial attachment after 24 h without any observation of biofilm formation [33]. The antibiofouling performance for a superhydrophobic surface is ascribed to the entrapped air layer, which can effectively reduce the contact area between bacteria and material surfaces, leading to reduced bacterial adhesion [34]. Therefore, superhydrophobicity has received increasing attention in the bacteriostatic field of titanium-based implants.
2. Interaction between Bacteria and Material Surfaces
To develop antibacterial superhydrophobic titanium-based implants, it is necessary to understand the interaction between bacteria and material surfaces. Bacteria are essentially single-celled organisms. However, they can attach to both inactive and active surfaces to create well-organized three-dimensional (3D) colonies that are known as biofilms in nature [35]. Biofilm formation is a multi-step (often periodic) process with several distinct stages, and the interaction between bacteria and material surfaces can be approximately separated into four steps [36], which are graphically summarized in Figure 1.
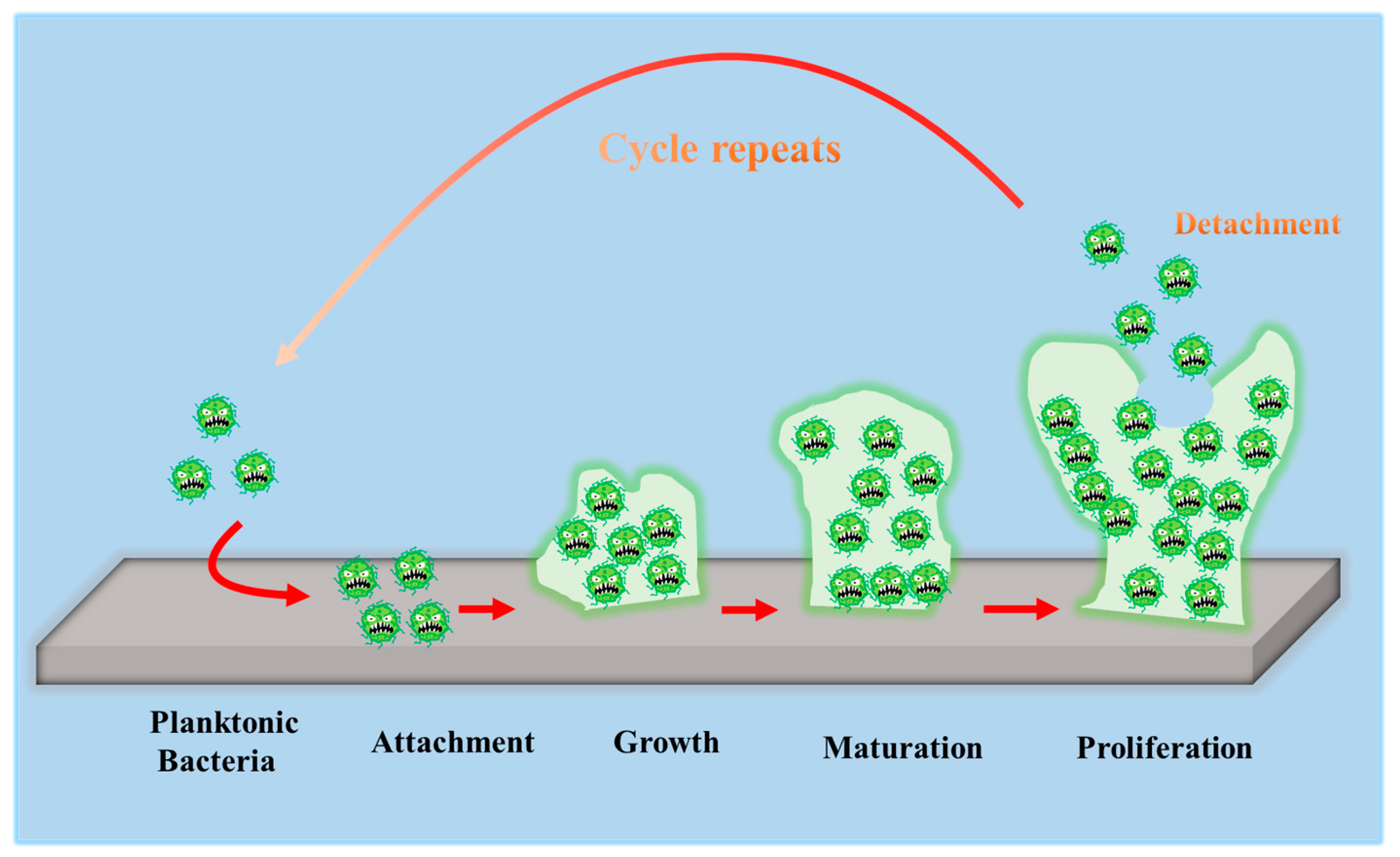
Figure 1. Schematic representation of biofilm formation.
-
The first step involves the bacterial attachment to material surfaces via cell-surface-associated adhesion [37][38][39]. The bacterial attachment to materials is primarily governed by steric interactions, electrostatic interplays, van der Waals forces, and protein adhesion, all of which are beneficial to making bacteria attach to the surface [40][41]. The process of bacterial attachment is invertible.
-
The subsequent step is the bacterial colonization on material surfaces, which is mediated through particular cellular and molecular interactions, such as adhesion proteins, protein appendages, and extracellular polymeric substance (EPS) generation [42]. The process of the bacterial colonization is nonreversible.
-
The third step is the formation and maturation of the biofilm. Bacteria that colonize surfaces will develop bacterial microcolonies and produce EPS (primarily polysaccharides and other macro-molecules), which can be helpful to biofilm formation. The maturation process includes EPS formation, cell agglomeration, chemical reactions, quorum sensing, and microcolony production. A biofilm will shield bacteria in a self-generated polysaccharidic matrix from the fluid shear force and protect the effects of systematic pharmaceutical treatments once it has grown on the surfaces [42].
-
The last step is bacterial proliferation. Bacteria start to proliferate under the protection of the biofilm on material surfaces. Consequently, bacteria will cover the surfaces entirely.
3. Antibacterial Applications
The most common causes of healthcare-associated infections (HCAIs) are Staphylococcus aureus (S. aureus) and Staphylococcus epidermidis (S. epidermidis), which are responsible for 31–52% of infections in orthopedic prostheses, 40–50% of infections in prosthetic heart valves, 50–70% of infections in prosthetic catheter biofilm, and 87% of infections in the bloodstream [43]. Pseudomonas aeruginosa (P. aeruginosa), a bacterium frequently present in soil and water, can also cause surgical site infections after hip replacement operations as well as chronic infections. Furthermore, P. aeruginosa has the ability to quickly form strong biofilms that can support other pathogenic types. Recent studies have pointed out that the appearance of bacterial biofilms is still a significant factor in triggering the implant looseness and hastening the failure of implant surgical procedures [44][45][46]. Once biofilm-associated infection occurs in clinics, secondary revision surgery will be unavoidable, resulting in additional pain, economic costs, and even significant psychological trauma for patients [47].
The most frequent cause of implant-related infections is attributed to Escherichia coli (E. coli) and S. aureus in the development of antibacterial titanium-based alloys [36]. The accumulation of bacterial biofilms on the implant surface is vulnerable to bacterial attachment, leading to peri-implant infection. When bacteria fully attach to the surface, the implant will most probably lose its effectiveness because biomaterial-associated infections (BAIs) are highly resistive to the innate immune system, antimicrobial agents, and chemotherapy drugs. The concept of antibacterial implants was introduced to titanium-based implants which are widely applied in orthopedic, dental, craniofacial surgeries and so on [48][49][50][51].
This entry is adapted from the peer-reviewed paper 10.3390/coatings13020419
References
- Wu, S.L.; Liu, X.M.; Yeung, K.W.K.; Guo, H.; Li, P.H.; Hu, T.; Chung, C.Y.; Chu, P.K. Surface nano-architectures and their effects on the mechanical properties and corrosion behavior of Ti-based orthopedic implants. Surf. Coat. Technol. 2013, 233, 13–26.
- Anene, F.A.; Aiza Jaafar, C.N.; Zainol, I.; Azmah Hanim, M.A.; Suraya, M.T. Biomedical materials: A review of titanium based alloys. Proc. Inst. Mech. Eng. Part C 2020, 235, 3792–3805.
- Boyan, B.D.; Bonewald, L.F.; Paschalis, E.P.; Lohmann, C.H.; Rosser, J.; Cochran, D.L.; Dean, D.D.; Schwartz, Z.; Boskey, A.L. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif. Tissue Int. 2002, 71, 519–529.
- Liu, X.; Lim, J.Y.; Donahue, H.J.; Dhurjati, R.; Mastro, A.M.; Vogler, E.A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials 2007, 28, 4535–4550.
- Schwartz, Z.; Raz, P.; Zhao, G.; Barak, Y.; Tauber, M.; Yao, H.; Boyan, B.D. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J. Bone Jt. Surg. Am. 2008, 90, 2485–2498.
- Wall, I.; Donos, N.; Carlqvist, K.; Jones, F.; Brett, P. Modified titanium surfaces promote accelerated osteogenic differentiation of mesenchymal stromal cells in vitro. Bone 2009, 45, 17–26.
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277.
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403.
- Yuan, Z.; He, Y.; Lin, C.C.; Liu, P.; Cai, K.Y. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51–67.
- D’Orto, B.; Polizzi, E.; Nagni, M.; Tete, G.; Cappare, P. Full arch implant-prosthetic rehabilitation in patients with type I diabetes mellitus: Retrospective clinical study with 10 year follow-up. Int. J. Environ. Res. Public Health 2022, 19, 11735.
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494.
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34.
- Ahiropoulos, V.; Helvatjoglu-Antoniades, M.; Papadogiannis, Y. In vitro fluoride uptake by bovine enamel from aesthetic restorative materials. Int. J. Paediatr. Dent. 2008, 18, 291–299.
- Evaristo, F.F.V.; de Vasconcelos, M.A.; Arruda, F.V.S.; Pereira, A.L.; Andrade, A.L.; de Alencar, D.B.; do Nascimento, M.F.; Sampaio, A.H.; Saker-Sampaio, S.; Bandeira, P.N.; et al. Antibacterial effect on mature biofilms of oral streptococci and antioxidant activity of 3β,6β,16β-trihydroxylup-20(29)-ene from Combretum leprosum. Med. Chem. Res. 2017, 26, 3296–3306.
- Liu, J.; Sun, L.; Liu, W.; Guo, L.; Liu, Z.; Wei, X.; Ling, J. A nuclease from streptococcus mutans facilitates biofilm dispersal and escape from killing by neutrophil extracellular traps. Front. Cell. Infect. Microbiol. 2017, 7, 97–110.
- Ozturk, O.; Sudagidan, M.; Turkan, U. Biofilm formation by staphylococcus epidermidis on nitrogen ion implanted CoCrMo alloy material. J. Biomed. Mater. Res., Part A 2007, 81, 663–668.
- Schutzius, T.M.; Jung, S.; Maitra, T.; Graeber, G.; Kohme, M.; Poulikakos, D. Spontaneous droplet trampolining on rigid superhydrophobic surfaces. Nature 2015, 527, 82–85.
- Peng, C.; Chen, Z.; Tiwari, M.K. All-organic superhydrophobic coatings with mechanochemical robustness and liquid impalement resistance. Nat. Mater. 2018, 17, 355–360.
- Zheng, S.; Li, C.; Zhang, Y.; Xiang, T.; Cao, Y.; Li, Q.; Chen, Z. A general strategy towards superhydrophobic self-cleaning and anti-corrosion metallic surfaces: An example with aluminum alloy. Coatings 2021, 11, 788.
- Kurbanova, A.; Myrzakhmetova, N.; Akimbayeva, N.; Kishibayev, K.; Nurbekova, M.; Kanagat, Y.; Tursynova, A.; Zhunussova, T.; Seralin, A.; Kudaibergenova, R.; et al. Superhydrophobic SiO2/trimethylchlorosilane coating for self-cleaning application of construction materials. Coatings 2022, 12, 1422.
- Zheng, S.; Li, C.; Fu, Q.; Hu, W.; Xiang, T.; Wang, Q.; Du, M.; Liu, X.; Chen, Z. Development of stable superhydrophobic coatings on aluminum surface for corrosion-resistant, self-cleaning, and anti-icing applications. Mater. Des. 2016, 93, 261–270.
- Zheng, S.; Li, C.; Fu, Q.; Xiang, T.; Hu, W.; Wang, J.; Ding, S.; Liu, P.; Chen, Z. Fabrication of a micro-nanostructured superhydrophobic aluminum surface with excellent corrosion resistance and anti-icing performance. RSC Adv. 2016, 6, 79389–79400.
- Zheng, S.; Bellido-Aguilar, D.A.; Huang, Y.; Zeng, X.; Zhang, Q.; Chen, Z. Mechanically robust hydrophobic bio-based epoxy coatings for anti-corrosion application. Surf. Coat. Technol. 2019, 363, 43–50.
- Peng, B.; Hongliang, L.; Guochen, Z.; Minrui, R.; Lili, C.; Hanjie, G.; Yanpeng, X. Robust super-hydrophobic coating prepared by electrochemical surface engineering for corrosion protection. Coatings 2019, 9, 452.
- Shao, W.; Kan, Q.; Bai, X.; Wang, C. Robust superhydrophobic coatings for enhanced corrosion resistance and dielectric properties. Coatings 2022, 12, 1655.
- Wang, L.; Gong, Q.; Zhan, S.; Jiang, L.; Zheng, Y. Robust anti-icingg performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735.
- Shen, Y.; Wang, G.; Tao, J.; Zhu, C.; Liu, S.; Jin, M.; Xie, Y.; Chen, Z. Anti-icing performance of superhydrophobic texture surfaces depending on reference environments. Adv. Mater. Interfaces 2017, 4, 1700836.
- Gao, S.; Dong, X.; Huang, J.; Li, S.; Li, Y.; Chen, Z.; Lai, Y. Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem. Eng. J. 2018, 333, 621–629.
- Zhang, C.; Yang, Y.; Luo, S.; Cheng, C.; Wang, S.; Liu, B. Fabrication of superhydrophobic composite membranes with honeycomb porous structure for oil/water separation. Coatings 2022, 12, 1698.
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8.
- Jiang, L.; Wang, R.; Yang, B.; Li, T.J.; Tryk, D.A.; Fujishima, A.; Hashimoto, K.; Zhu, D.B. Binary cooperative complementary nanoscale interfacial materials. Pure Appl. Chem. 2000, 72, 73–81.
- Xiang, T.F.; Lv, Z.; Wei, F.F.; Liu, J.; Dong, W.; Li, C.; Zhao, Y.X.; Chen, D.P. Superhydrophobic civil engineering materials: A review from recent developments. Coatings 2019, 9, 753.
- Hwang, G.B.; Page, K.; Patir, A.; Nair, S.P.; Allan, E.; Parkin, I.P. The anti-biofouling properties of superhydrophobic surfaces are short-lived. ACS Nano 2018, 12, 6050–6058.
- Bartlet, K.; Movafaghi, S.; Dasi, L.P.; Kota, A.K.; Popat, K.C. Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surf. B 2018, 166, 179–186.
- Banerjee, D.; Shivapriya, P.M.; Gautam, P.K.; Misra, K.; Sahoo, A.K.; Samanta, S.K. A review on basic biology of bacterial biofilm infections and their treatments by nanotechnology-based approaches. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 90, 243–259.
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612.
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755.
- Pirrone, M.; Pinciroli, R.; Berra, L. Microbiome, biofilms, and pneumonia in the ICU. Curr. Opin. Infect. Dis. 2016, 29, 160–166.
- Harriott, M.M. Biofilms and antibiotics. In Reference Module in Biomedical Sciences; Caplan, M., Mitchell, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–11.
- Stoica, P.; Chifiriuc, M.C.; Rapa, M.; Lazăr, V. Overview of biofilm-related problems in medical devices. In Biofilms and Implantable Medical Devices; Deng, Y., Lv, W., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–23.
- Rimondini, L.; Cochis, A.; Varoni, E.; Azzimonti, B.; Carrassi, A. Biofilm formation on implants and prosthetic dental materials. In Handbook of Bioceramics and Biocomposites, 1st ed.; Antoniac, I.V., Ed.; Springer: Cham, Switzerland, 2016; pp. 991–1027.
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978.
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067.
- Tan, L.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Zhu, S.; Li, Z.; Yuan, X.; Zheng, Y.; Yeung, K.W.K.; et al. Rapid biofilm eradication on bone implants using red phosphorus and near-infrared light. Adv. Mater. 2018, 30, 1801808.
- Li, M.; Li, L.; Su, K.; Liu, X.; Zhang, T.; Liang, Y.; Jing, D.; Yang, X.; Zheng, D.; Cui, Z.; et al. Highly effective and noninvasive near-infrared eradication of a staphylococcus aureus biofilm on implants by a photoresponsive coating within 20 min. Adv. Sci. 2019, 6, 1900599.
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplast. 2012, 27, 61–65.
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 470–480.
- Mu, X.; Yan, C.; Tian, Q.; Lin, J.; Yang, S. BSA-assisted synthesis of ultrasmall gallic acid-Fe(III) coordination polymer nanoparticles for cancer theranostics. Int. J. Nanomed. 2017, 12, 7207–7223.
- Han, J.M.; Hong, G.; Hayashida, K.; Maeda, T.; Murata, H.; Sasaki, K. Influence of composition on the adhesive strength and initial viscosity of denture adhesives. Dent. Mater. J. 2014, 33, 98–103.
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; Von Eiff, C. Silver-coated megaendoprostheses in a rabbit model–an analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556.
- Arciola, C.R.; Campoccia, D.; Ehrlich, G.D.; Montanaro, L. Biofilm-based implant infections in orthopaedics. Adv. Exp. Med. Biol. 2015, 830, 29–46.
This entry is offline, you can click here to edit this entry!
