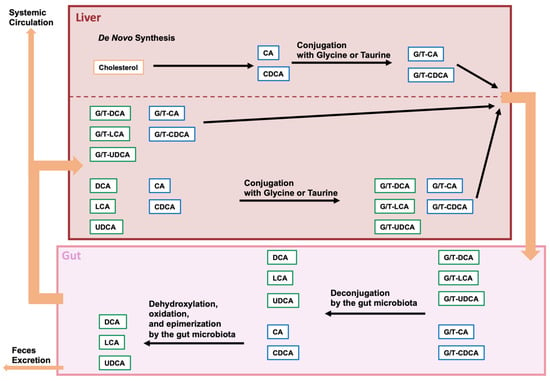
In the intestine, bile salt hydrolase (BSH) deconjugates conjugated BAs. Various gut bacteria have been shown to exhibit BSH activity [
]. Most of these bacteria, such as
]. After deconjugation, the 7α-hydroxy group is removed from CA and CDCA, leading to a yield of deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. A few bacteria among
spp. have been identified as capable of eliminating the 7α-hydroxy group. The α-dehydroxylation of the CA and CDCA is carried out by enzymes that are encoded in the bile acid-inducible (
) operon.
The composition of gut microbiota and BAs is affected by diet, age, sex, antibiotics, and disease [
7,
28,
29,
30]. BAs and gut microbiota affect each other [
5,
6]. BAs are modified by gut bacteria through a variety of enzymatic reactions. Thus, the diversity of gut bacteria involved in the modification of bile acids has implications for host physiology and pathophysiology. Bacteria harboring BSH have been reported to be widely distributed across bacterial phyla and approximately 26% of bacteria strains in the Human Microbiome Project [
31]. However, bacteria capable of conducting 7-α-dehydroxylation is limited to
Clostridium spp. [
27,
32,
33]. Furthermore, changes in the levels of microorganisms in gut microbiota are associated with neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease [
34,
35]. The relationship between gut microbiota and various diseases is currently being studied using multi-omics analysis, including metagenomics, metatranscriptomics, metaproteomics, and metabolomics [
36]. There are two major types of metagenomic analyses that comprehensively analyze microbial communities using next-generation sequencers: 16S rRNA gene sequencing and shotgun metagenomic sequencing; these identify microorganisms and evaluate diversity and abundance [
37]. 16S rRNA gene sequencing is the most widely used method of analyzing gut microbiota, and it is relatively inexpensive and simple to perform. In the 16S rRNA gene sequencing metagenomic analysis, after PCR amplification is conducted to target the 16S rRNA genes, the amplified PCR products are comprehensively sequenced using next-generation sequencing (NGS) to identify the diversity of gut microbiota and the types and composition ratios of its constituent bacteria. In shotgun metagenomic sequencing, DNA is randomly split into fragments, which are then sequenced by NGS. The sequenced DNA is linked using bioinformatics, resulting in the identification of species, strains, and functional genes [
38,
39]. These metagenomic analyses have revealed the association between changes in the composition of the gut microbiota and neurodegenerative diseases; furthermore, but their means, the association between changes in bacterial species with enzymes that modify BAs and neurodegenerative diseases are also being elucidated [
34,
35]. In patients with Alzheimer’s disease, Bacteroidetes is positively correlated with Alzheimer’s disease, while Firmicutes and
Bifidobacterium are negatively correlated with Alzheimer’s disease [
40,
41,
42]. In patients with Parkinson’s disease,
Akkermansia is positively correlated with Parkinson’s disease, while
Lactobacillus is negatively correlated with Parkinson’s disease [
43,
44,
45]. In patients with Huntington’s disease,
Intestinimonas,
Bilophila,
Lactobacillus,
Oscillibacter,
Gemmiger, and
Dialister are positively correlated with Huntington’s disease [
46] and Firmicutes,
Lachnospiraceae, and
Akkermansiaceae are negatively correlated with Huntington’s disease gene expansion carriers [
47].
3. Microbiota-Modified BAs in Neurodegenerative Diseases
The source of the BAs found in the brain has not been identified, but conjugated and unconjugated BAs have been found there [
102,
103,
104]. BAs present in the brain may be produced there or migrate through the circulatory system. Primary BAs (CA and CDCA) are also produced by de novo synthesis in the brain. These primary BAs are responsible for the majority of cholesterol metabolism in the brain. This third pathway in the brain to produce primary BAs by de novo synthesis is called neural pathway [
105]. Because secondary BAs are generated by the gut microbiota, the secondary BAs detected in the brain are thought to be transferred from the circulation. Furthermore, because a correlation has been identified between brain bile acid concentrations and serum bile acid concentrations, it is now believed that the majority of BAs in the brain migrate through circulation [
106,
107]. However, because there is neural pathway to BA synthesis, primary BAs produced by de novo synthesis in the brain may also play a role in the physiological and pathophysiological condition [
105]. BAs are transported from the circulation, either through the blood–brain barrier (BBB) or through BA transporters [
16,
108]. Lipophilic BAs can pass through the BBB through passive diffusion. By contrast, hydrophilic BAs can pass through the BBB through transporters [
109,
110]. Therefore, the brain can be influenced by the gut microbiota via BAs.
3.1. Alzheimer’s Disease
Alzheimer’s disease is a progressive and irreversible neurodegenerative disease characterized by dementia, memory loss, and morphological changes in multiple regions of the brain. The pathological features of patients with Alzheimer’s disease are the accumulation of amyloid β peptide and tau protein entanglement in the brain [
111]. Amyloid β peptide is produced from amyloid precursor protein (APP) with β- and γ-secretase [
112,
113]. The γ-secretase complex contains presenilin 1 (PS1). Autophagy refers to the process of removing the accumulation of misfolded proteins, and the suppression of autophagy is connected with Alzheimer’s disease [
114]. Therefore, BAs can affect Alzheimer’s disease by influencing autophagy. LCA levels in plasma are higher in patients with Alzheimer’s disease. In addition, LCA levels in plasma increase by approximately 3 fold within 8–9 years from when healthy subjects develop Alzheimer’s disease [
115]. By contrast, the levels of CA in plasma and the TCA levels in the brain are significantly lower in patients with Alzheimer’s disease [
104]. However, the neuroprotective effect of TUDCA, which is a taurine-conjugated secondary BA, has been demonstrated in neurodegenerative disease [
116]. TUDCA lowers amyloid β peptides and relieves memory deterioration in APP/PS1 double-knockout mice used as a model for Alzheimer’s disease [
117,
118]. The ratios for both unconjugated and conjugated secondary BAs, including LCA, GCDCA, taurodeoxycholic acid (TDCA), glycodeoxycholic acid (GDCA), and UDCA, to CA were higher in the brain of patients with Alzheimer’s disease [
102].
3.2. Parkinson’s Disease
Parkinson’s disease is a progressive neurodegenerative disease that manifests itself in resting tremor, muscle rigidity, bradykinesia, akinesia, and postural reflex disorder [
122]. It results in the loss of dopaminergic neurons in the substantia nigra [
123]. The dysfunction of phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1) and Parkin is considered a major cause of parkisonisms [
124], and this dysfunction results in the impairment of mitophagy, suggesting that the quality control of mitochondria plays an crucial role in the suppression of Parkinson’s disease [
61,
125]. BAs are associated with autophagy and the quality control of mitochondria. Furthermore, plasma levels of CA, DCA, TDCA, and GDCA in patients with Parkinson’s disease are significantly higher when compared with healthy subjects [
126,
127]. However, the plasma levels of GUDCA in patients with Parkinson’s disease are decreased [
128].
3.3. Huntington’s Disease
Huntington’s disease is an autosomal-dominant neurodegenerative disease that manifests in cognitive disability, and psychiatric disturbance, and motor dysfunctions [
135]. Huntington’s disease is caused by cytosine-adenine-guanine (CAG) expansion which encodes a polyglutamine at the N-terminus of huntingtin (HTT) [
136]. HTT has a similar structure to the three autophagy proteins of yeast, Atg11, Atg23, and Vac8 [
137,
138] and acts as an autophagy initiator and enhancer [
139]. The mutation of HTT leads to a reduction in mitophagy [
140]. BAs are associated with mitophagy. In addition, 3-nitropropionic acid (3-NP) selectively damages neurons in the striatum and is involved in the development of Huntington’s disease. 3-NP inhibits succinate dehydrogenase in mitochondria and leads to the degeneration of the caudate-putamen [
141,
142]. TUDCA improves 3-NP-induced neural mitochondrial damage, neural cell death, and sensorimotor deficits [
143].