Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Thyroid cancer is the most common endocrine malignancy, with an increasing trend in the past decades. It has a variety of different histological subtypes, the most frequent one being differentiated thyroid cancer, which refers to papillary carcinoma, the most common histological type, followed by follicular carcinoma. Associations between genetic polymorphisms and thyroid cancer have been investigated over the years and are an intriguing topic for the scientific world.
- thyroid cancer
- differentiated thyroid cancer
- thyroid cancer prognosis
- molecular biomarkers
- targeted therapy
- single nucleotide polymorphism
- genetic variation
- genetic predisposition
1. Introduction
Thyroid cancer, the most frequent endocrine malignancy, has recorded an increase in global incidence in recent decades. This increase is mostly attributed to the increased detection of low-risk tumors through intensive screening, which may result in an overdiagnosing phenomenon [1]. Papillary thyroid carcinoma (PTC) is the most common histological type, followed by follicular thyroid carcinoma (FTC). The papillary and follicular histological types are known as differentiated thyroid cancer (DTC) to distinguish them from poorly differentiated thyroid and anaplastic carcinomas, which originate from the follicular tissue, as well as from medullary thyroid cancer, which is derived from the parafollicular C cells [2]. Several risk factors, modifiable and non-modifiable, have been linked to an increased likelihood of developing thyroid cancer (Figure 1) [3].
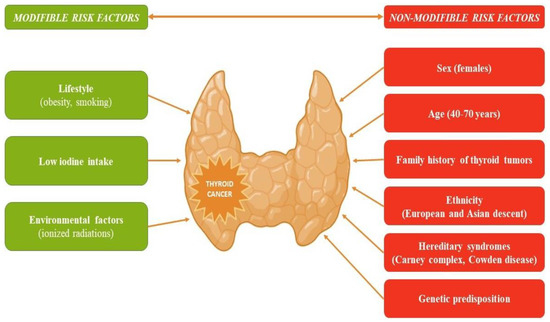
Figure 1. Risk factors that increase the likelihood of developing thyroid cancer.
The prognosis in DTC is often favorable, but it is influenced by the tumor’s histological type. PTC has an excellent prognosis, although a small proportion of patients show an aggressive, unfavorable evolution [4][5]. In recent years, molecular markers such as B-Raf Proto-Oncogene, Serine/Threonine Kinase (BRAF) V600E and Neuroblastoma RAS viral oncogene homolog (NRAS) mutations, and more recently, mutations in telomerase reverse transcriptase promoter (pTERT), have been studied for their role as predictors in treatment response, as well as their association with various clinico-pathological features, recurrence, and mortality in patients with DTC [6][7]. The co-existence of pTERT and the BRAF V600E mutation is associated with an unfavorable prognosis, distant metastases, and increased mortality [8][9]. As opposed to genetic mutation, which is defined as any abnormal change in a DNA sequence, a genetic polymorphism is a DNA sequence variation commonly found in the population [10].
The identification of patients diagnosed with thyroid cancer at risk of having an unfavorable prognosis has great importance in optimizing individual therapeutic management. Researchers aim to emphasize the existing literature data regarding genetic polymorphisms investigated for their potential association with DTC and highlight the opportunity of using genetic variations as biomarkers of diagnosis and prognosis for thyroid cancer patients.
2. SNPs of Tyrosine-Kinase Family Genes
2.1. SNPs of RET Gene
The Rearranged during Transfection (RET) protooncogene encodes a receptor of the tyrosine-kinase proteins and has been associated with numerous types of cancers, including thyroid cancer. The process leading to carcinogenesis implies gain-of-function mutations that translate to RET activation [11]. Santos et al. conducted a case-control study in 2014 on a total of 1088 individuals (545 with DTC and 543 controls) to determine the effect of four SNPs (G691S, L769L, S836S, and S904S) of RET in the risk for DTC. It was noted that RET S836S is overexpressed in patients with PTC as well as the GGTC haplotype, suggesting that it might be a risk factor for PTC. Interestingly, the overrepresentation of minor alleles G691S and S904S was identified in an almost significant percentage of tumors >10 mm at diagnosis, hinting at a possible role in tumor behavior. No such associations were found in FTC [12]. On the other hand, He et al. noted that the haplotype CGGATAA of rs17028, rs1799939, rs1800858, rs1800860, rs2075912, rs2565200, and rs2742240 was associated with a reduced risk of DTC (OR = 0.18, p = 0.001) in a candidate gene association study conducted on 552 subjects (300 with DTC and 252 controls). In the same study, rs1799939 AG or AG plus AA genotypes were associated with increased risk for DTC without concomitant thyroid benign disorders (OR = 1.93, p = 0.009 and OR = 1.88, p = 0.011, respectively). Moreover, an increased risk for distant metastases was found for haplotype CAAGCGT of rs17028, rs1799939, rs1800858, rs1800860, rs2075912, rs2565200, and rs2742240 (OR = 7.57, p = 0.009) [13].
Although this research offers interesting information, the study population involved in both studies was relatively small, therefore the impact of RET SNPs on DTC risk might be underestimated. Furthermore, neither one of the studies assessed radiation exposure, a factor that has a great impact on the oncogenesis of thyroid malignancy. A recent meta-analysis showed strong epidemiological evidence of associations through the Venice criteria and false-positive report probability between thyroid cancer susceptibility and RET rs1799939 [14].
2.2. SNPs of MET Gene
The cellular mesenchymal-epithelial transition (MET) factor is a plasma membrane tyrosine kinase receptor with low activity in normal cells, which may become activated in tumor cells through mutations, amplifications, or overexpression, leading to a potential increase in the aggressiveness of cancer [15][16]. The role of MET gene SNPs in PTC was investigated by Ning et al., who evaluated 858 patients with PTC [17]. MET SNP (rs1621) showed a significant association with PTC in females. Moreover, the rs1621 AG genotype might be especially associated with the female sex as it was significantly higher in the PTC group for female patients and had an increased risk of PTC. On the other hand, the analyzed MET SNPs revealed no correlation with metastasis and prognosis, regardless of gender [17].
3. SNPs of Genes Involved in Apoptosis, Genome Stability, and DNA Repair
3.1. SNPs of BAX Gene
B-cell lymphoma 2-associated X protein (BAX) has an important role in mitochondrial apoptosis [18]. There might be an association between BAX gene polymorphism and oncogenesis, mainly due to the −248 G > A polymorphism that down-regulates the BAX gene transcription which ultimately might inhibit the apoptosis in tumoral cells [19]. However, a meta-analysis from 2013 suggested that BAX −248 G > A polymorphism is not to be considered an important oncogenic factor [20]. As for research which assessed this polymorphism and DTC, Cardoso-Duarte et al. recently conducted a case-control study on 30 Brazilian patients with PTC and concluded that the BAX single nucleotide polymorphism −248 G > A GG genotype was associated with PTC and the presence of the G allele was a protective factor against the occurrence of PTC [21].
3.2. SNPs of TP53 and p21 Gene
It is well known that events leading to DNA damage critically raise the risk of developing cancer. Tumor protein 53 (TP53) has a main role in protecting the genome from getting damaged; therefore, it plays an important part in cancer development, including thyroid cancer [22]. Depending on the severity of DNA damage, TP53 determines if the cell undergoes a repairing process or apoptosis [23]. The p21 protein is a cyclin-dependent kinase inhibitor that is expressed by the activated TP53 during the G1 phase of the cell cycle, inhibiting DNA replication and therefore stopping the progression of the cell cycle [24]. Therefore, there are data stating that in more than half of all cancers, TP53/p21 is inactivated [25].
Heidari et al. investigated if SNPs in TP53 (rs1042522) and p21 (rs1059234 and rs1801270) genes affect the risk of PTC or if they are associated with clinical and histopathological features of PTC. The study was designed as a case-control study and found that the TP53-rs1042522 CC genotype was significantly associated with protection against PTC in the dominant, recessive, and allelic models (p = 0.008, p = 0.01, p = 0.002, respectively). Moreover, the rs1042522 was associated with tumors > 1 cm in dominant and recessive models (p = 0.04, p = 0.009, respectively) and with vascular invasion in the dominant model (p = 0.01). However, regarding SNP of the p21 gene (rs10559234 and rs1801270), no correlation was found with the risk of PTC or clinical and histopathologic features [26]. There is little data in the literature concerning SNP in these genes and their role in thyroid cancer; therefore, there is a need to expand the research towards this area knowing the role they have in suppressing carcinogenesis.
3.3. SNPs of HOTAIR Gene
SNPs in the HOX Transcript Antisense RNA (HOTAIR) gene, which is an oncogene that regulates gene expression and chromatin dynamics, have been associated with breast and colorectal cancers [27][28]. Regarding thyroid cancer, there are mixed results depending on the SNP studied. Rad et al. found that HOTAIR rs1899663 gene polymorphism was not associated with clinical or histopathological features of thyroid cancer [29]. On the other hand, Min et al. showed that HOTAIR rs12826786 and rs920778 had an increased thyroid cancer risk, whereas rs7958904, rs4759314, rs874945, and rs189963 did not correlate with increased thyroid cancer risk [30].
3.4. SNPs of XRCC1 Gene
The X-ray repair cross-complementing group 1 (XRCC1) proteins have a central role in the base excision repair, an essential DNA repair pathway, thus preserving the genome stability [31]. In the past years, the role of the XRCC1 Arg194Trp polymorphism in the development of DTC has been investigated. A meta-analysis published in 2016 by Zhao JZ et al. pointed out the XRCC1 Arg194Trp polymorphism had an increased thyroid cancer risk in the Caucasian population [32]. On the other hand, in a more recent study published by Liu SY that also investigated XRCC1 Arg194Trp polymorphism and its association with susceptibility to thyroid cancer, the C allele of XRCC1 had an 18% significantly decreased risk of thyroid cancer in Chinese people, but without any association among Caucasians [33].
4. SNPs of the VDR Gene
Low vitamin D levels were correlated with an increased risk of advanced papillary thyroid cancer in several studies, being associated with local or distant metastasis and having a potential prognostic impact [34][35][36]. Vitamin D receptor (VDR) polymorphisms might increase the risk of several types of cancer (breast, ovarian, colorectal) [37][38][39].
Regarding thyroid cancer, Beysel et al. published a case-control study in 2018 and found that the VDR gene FokI (rs2228570) CT/TT genotype had an increased risk of PTC, whereas the FokI TT genotype usually presented with increased tumor diameter (T3 and T4), advanced stage (III/IV), and extra-thyroidal invasion. Moreover, the FokI CT/TT or TT genotypes were associated with lymph node metastasis, multifocality, and tumors >10 mm [40]. Another study conducted on Romanian patients with DTC investigated the correlation between vitamin D levels, VDR gene polymorphisms, clinical findings, and histopathological traits and found that vitamin D levels were significantly lower in patients with DTC and that FokI polymorphisms were frequently encountered in patients with DTC. Moreover, the Ff genotype was associated with more aggressive forms [41].
These studies showed promising results and future research should focus on studying in a more detailed manner the opportunities of using FokI as a poor prognostic factor in DTC.
5. SNPs of Extracellular Matrix Genes with Roles in Cellular Proliferation and Differentiation
5.1. SNPs of SPARC and SPP1 Gene
Matricellular proteins such as secreted phosphoprotein 1 (SPP1) and secreted protein acidic and rich in cysteine (SPARC) are involved in preserving cellular stability and structure [42]. The aberrant expression of these proteins might lead to several types of cancer progression or metastases [43][44]. Su X et al. noted that three loci, rs1054204, rs3210714, and rs3549 in SPARC and rs4754 in SPP1, individually or combined, are involved in PTC susceptibility, either decreasing or increasing the risk of developing the disease [45]. Currently, data regarding genetic variants of matricellular proteins in thyroid cancer are scarce, therefore there is great potential to explore this research area through future studies.
5.2. SNPs of MMP-9 Gene
Matrix metalloproteinases, also knowns as matrixins, are calcium-dependent and zinc-containing endoproteinases, which are capable of degrading numerous extracellular matrix proteins and processing several types of bioactive molecules, having an important part in processes such as cell proliferation, adhesion/dispersion, angiogenesis, and apoptosis [46]. Matrix metalloproteinase-9 (MMP-9) is one of the most important and complex MMPs, with implications in inflammatory activity, immune response, activation of tumor growth factor β in cancer progression, and resistance of tumor cells, which has a strong application as a cancer growth, invasion, and metastasis mediator [47]. MMP-9 has a physiologically low-level expression but is overexpressed in several types of cancers [47][48].
Serum MMP-9 levels measured using an immunometric assay were evaluated in subjects with PTC in a case-control study that concluded that although serum MMP-9 is not helpful in the diagnosis of PTC, a high pre-surgical level might be a prognostic factor that could dictate the treatment aggressiveness [49]. In a recent study that investigated the role of MMP-9 promoter −1562C/T functional SNP in the risk of developing PTC, the T allele was found significantly more frequently in subjects with PTC (17.5% vs. 10.1%, p = 0.019) [50]. PTC had an increased risk of developing in subjects with the CT or CT + TT genotype. Although MMP-9 promoter SNP seems to increase the susceptibility of developing PTC, it was not associated with tumor histological subtype, invasion, or tumor stage [50]. Nevertheless, despite these promising results that strongly suggest a role for MMP-9 SNPs in thyroid carcinogenesis, future research is still needed on larger populations.
5.3. SNPs of NRG1 Gene
The neuregulin 1 (NRG1) gene encodes a glycoprotein that mediates cell–cell signaling. It is produced in multiple isoforms and plays an important part in the proliferation, survival, and differentiation of various system organs [51]. Several studies demonstrated an association between NRG1 rs2439302 and PTC [51][52][53]. Moreover, a recent meta-analysis consolidated the previous results stating that the SNP rs2439302 variants of the gene encoding NRG1 carry a high thyroid cancer risk, especially in Chinese populations compared to Japanese populations or populations from the United States of America [54].
6. SNPs of Genes Involved in Thyroid Morphogenesis and Function
6.1. SNPs of FOXE1 Gene
The Forkhead factor E1 (FOXE1) gene is a thyroid transcription factor and a member of the forkhead/winged-helix family with a crucial role in thyroid morphogenesis. It is also a key factor in follicular cell proliferation and differentiation, thus playing an important part in thyroid tumorigenesis [55]. FOXE1 rs965513 SNP offered an increased risk for DTC in a German population studied in a case-control study from 2014 conducted by Penna-Martinez et al., including in advanced tumor staging [56]. A meta-analysis published in 2018 by Chen YH et al. that combined the results of 15 studies found that common variants of FOXE1 rs965513, rs944289, and rs1867277 were risk factors for increased susceptibility to developing DTC [57]. However, the previously mentioned study was conducted mainly on Caucasians, with a small sample of Asian studies. Recently, a case-control study conducted in Iran on 81 patients with thyroid cancer and 165 controls found that FOXE1 polymorphism (rs1867277) could be used as a biomarker for thyroid cancer diagnosis, being found in 32.1% (homozygous GG) and 18.5% (homozygous AA) of thyroid cancer patients [58].
6.2. SNPs of TSH-β Gene
The thyroid-stimulating hormone (TSH) has two distinct subunits, namely the alpha subunit (TSH-α), which is common to all glycoprotein hormones, and the beta subunit (TSH-β), which ensures a normal functioning TSH [59][60]. Given that the TSH level influences thyroid function, genetic changes in the synthesis pathways of TSH might lead to thyroid diseases, including thyroid cancer. Mutations in the TSH-β gene might be linked to several types of thyroid dysfunctions, such as congenital central hypothyroidism or severe isolated TSH deficiency [61][62]. However, the TSH-β gene has an unclear role in the pathogenesis of thyroid cancer with little data in the literature. SNPs in the TSH-β gene and their role in the development and evolution of thyroid cancer have been investigated quite recently. A prospective case-control study conducted on 507 Saudi patients with DTC pointed out that TSH-β gene polymorphism (rs201857310) is associated with the disease and might have a role in its pathogenesis in this population [63]. Nevertheless, larger studies are required to verify this finding, expanding the research towards diverse ethnicities and analyzing the exposure to distinct environmental factors as well.
7. SNPs of Genes Involved in Folate Metabolism
Folate metabolism is known to be implicated in various processes such as synthesis, methylation, and DNA repair, with some genes such as methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), reduced folate carrier 1 (RFC1), and cystathionine β-synthase (CßS) having an important part in regulating this metabolism [64]. Genetic alterations in these genes could potentially lead to genetic instability, therefore increasing the risk of carcinogenesis.
Several studies in the literature state that genetic SNPs involved in folate metabolism could increase the likelihood of developing several types of cancer [65][66][67][68]. A study published in 2019 by Zara-Lopes et al. investigated the associations between MTHFR 677C > T, MTR 2756A > G, and RFC1 80A > G polymorphisms and thyroid cancer, also evaluating the association between these SNPs, risk factors, and clinico-histopathological features [64]. The study revealed an association between MTHFR 677C > 7 and thyroid cancer under the dominant, codominant, and recessive models. Furthermore, RFC1 80A > G was also associated with thyroid cancer, but only under the recessive model, whereas MTR 2756A > G showed an association with tumor size and aggressive behavior [64].
Based on these results, larger studies focusing on MTHFR 677C > 7 and MTR 2756A > G SNPs should be conducted on different populations to fully assess the potential of these SNPs as prognosis factors.
8. SNPs of Genes Involved in Inflammation
Nowadays, inflammation is considered a hallmark of cancer. The role of inflammation in cancer has been investigated by numerous researchers and was linked to cancer occurrence, metastasis, or treatment resistance. Therefore, targeting genes involved in the inflammatory process might have a great impact on cancer management.
Cytokines, especially several interleukins, play a critical role in the interaction between immune and non-immune cells in the tumor microenvironment, having implications for cancer development and progression [69]. Other molecules, such as nitric oxide, have an anti-inflammatory effect in physiological conditions, but can develop pro-inflammatory action in pathological situations [70]. Researchers aimed to briefly summarize the most recent results regarding SNPs in inflammation-related genes and thyroid cancer.
8.1. SNPs of IL-10 Gene
It is widely known that cytokines influence the activation, growth, and differentiation of various cells, also having a potential role in cancer occurrence. Tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) have a pivotal role in initiating the inflammatory response. Moreover, they are secreted by several types of cancer to promote tumoral growth [71]. On the other hand, interleukin-10 (IL-10) is an immunosuppressive cytokine that can stimulate cancer growth by helping the malignant cells escape the immune response [72]. In 2014, Cil et al. published a case-control study conducted on 190 patients with thyroid cancer and 216 controls which investigated the association between TNF-α G-308A, IL-6 G-174C, and IL-10 G-1082A SNP and clinico-biochemical features of PTC [73]. They found that SNPs of TNF-α G-308A and IL-6 G-174C had no impact on the risk of developing PTC. However, the frequency of the IL-10 1082-G allele was higher in the PTC group. Moreover, subjects with the IL-10 G-1082 GG genotype had an increased risk of developing PTC compared to the AA genotype, concluding that SNP in the IL-10 gene might have an important role in the pathogenesis of PTC [73].
8.2. SNPs of IL-1 Gene
The interleukin-1 (IL-1) system encompasses different cytokines that intervene in both innate inflammation and immune responses by acting upon two different subtypes of receptors: IL-1 receptor type 1 (IL1R1) and IL-1 receptor type 2 (IL1R2) [74]. Their genetic polymorphism seems to be linked to various autoimmune diseases, and sparsely, with inconclusive results, with PTC [75][76][77][78]. Xiong et al. published their results in 2019 after investigating the role of the IL1R1 and IL1R2 SNPs in thyroid cancer. They found that rs3917225 in IL1R1 as well as rs2072472 and rs11674595 in IL1R2 were linked to susceptibility to thyroid cancer [79].
Interleukin 1 alpha (IL1A) and interleukin 1 beta (IL1B) belong to the IL-1 protein cluster and are involved in chronic inflammation, as well as in cancer development [74]. In their paper, Li H et al. tried to determine whether IL1A and IL1B polymorphisms predispose to a higher risk of thyroid cancer. Twelve SNPs of IL1A and IL1B were examined, concluding that six of them (rs3783521, rs3783546, rs3783550, and rs1609682 for IL1A and rs3136558 and rs1143623 for IL1B) were linked to an increased susceptibility to thyroid cancer development. IL1B polymorphisms were associated with an increased thyroid cancer risk after the fifth decade of life (>48 years old), whereas IL1A polymorphisms and thyroid cancer susceptibility were linked to age below this threshold [80].
8.3. SNPs of NOS3 Gene
Nitric oxide is a radical that acts as a biological mediator in various processes in the body, being involved both at the level of macrovascularization and microvascularization. At the level of macrovascularization, nitric oxide’s function consists of suppressing inflammation and cell adhesion, inhibiting the thrombosis process, and improving blood flow. In terms of microvasculature, nitric oxide at this level promotes angiogenesis [81]. Nitric oxide has also been associated with modulating neuronal activity as well as with various types of cancer (breast cancer, melanoma metastases) [81]. Genetic variations in the nitric oxide synthase 3 (NOS3) gene can alter plasma nitric oxide concentrations while increasing oxygen free radical production [73]. Increased oxygen-free radicals can lead to oxidative stress with damage to DNA and RNA, increasing the risk of cancer [81][82].
As for its role in thyroid cancer, SNPs of the NOS3 gene were studied in an observational case-control study conducted on a Brazilian population of 31 patients diagnosed with PTC, who were operated on and treated with radioiodine (I131), and 81 healthy controls [83]. There was a significant genotype difference between the PTC group and the control group (p = 0.001). Furthermore, the BB genotype was considered a protective factor for PTC (p = 0.001), whereas the presence of the A allele appeared to be a risk factor (p = 0.001). The results also suggested that the polymorphism of the NOS3 gene in intron 4 might increase the susceptibility for papillary thyroid carcinoma, though without detecting any association with patients’ clinical and biological characteristics [83]. There is very little data in the literature regarding genetic variations in the NOS3 gene, therefore this topic should be addressed in future research considering the increased risk of malignancy that SNPs in this gene might be associated with.
9. SNP in lncRNAs
Approximately 75% of the human genome is transcribed in RNAs, with less than 2% of the genes being translated into proteins, meaning that approximately 98% of RNAs are involved at the transcriptional levels and are composed of non-coding RNAs (ncRNAs). These ncRNAs are classified as short ncRNAs or long non-coding RNAs (lncRNAs) [84][85]. The miRNAs are small, non-coding RNAs that regulate gene expression, and their dysfunction alters the expression of oncogenic or tumor-suppressor target genes [86]. Recently, Khan et al. demonstrated a strong association between miRNA-149 (MIR149) gene SNP rs2292832 and thyroid cancer, specifically the mutation T > C (p = 0.0004) [87]. Furthermore, Khan et al. observed that miRNA-34b/c (MIR34B) gene SNP rs4938723 had a strong association with thyroid cancer occurrence [88].
As for lncRNAs, they have received great cancer research interest in the past years. Numerous studies have demonstrated that these molecules are involved in various physiological and pathological processes, including carcinogenesis or metastasis. These processes play a critical role in the regulation of gene expression, chromatin remodeling, transcription, and post-transcriptional processes. At the same time, certain lncRNAs can play the role of tumor suppressors or oncogenes in various cancers [85].
Regarding thyroid cancer, in 2016, Yang et al. found 675 differentially expressed lncRNAs in PTC, out of which 312 were upregulated and 363 downregulated [89]. Some important lncRNAs studied over the years in thyroid cancer include Nuclear Paraspeckle Assembly Transcript 1 (NEAT1), HOTAIR, GAS8 Antisense RNA 1 (GAS8-AS1), Papillary Thyroid Carcinoma Susceptibility Candidate 3 (PTCSC3), Maternally Expressed 3 (MEG3), BRAF-Activated Non-Protein Coding RNA (BANCR), cancer susceptibility candidate 2 (CASC2), and Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) [90]. As for investigating lncRNAs SNPs and thyroid cancer, several studies have investigated possible links between lncRNAs SNPs and PTC, establishing, for example, that lncRNAs BANCR, PTCSC3, and MEG3 are downregulated and have tumor suppressor roles in thyroid cancer, whereas lncRNAs CDKN2B Antisense RNA 1 (ANRIL), MALAT1, HOTAIR, and HIT000218960 are upregulated and function as oncogenes [91][92][93][94][95][96][97][98]. Huang et al. investigated the effects of lncRNA CASC2 in PTC. They found that the overexpression of CASC2 promoted inhibition of carcinogenesis in PTC, suggesting that CASC2 might have a potential role as a prognostic or therapeutic biomarker [99]. A possible novel therapeutic biomarker could be the lncRNA Copy Number Amplified Long noncoding RNA in Papillary Thyroid Cancer 1 (CNALPTC1), which was named and investigated by Chen et al. in 2018. They found that CNALPTC1 was upregulated in PTC and its overexpression was associated with aggressive behavior [100].
lncRNAs SNPs represent a new and exciting cancer research area. Several new studies have investigated lncRNAs SNPs and various results have been reported in recent years, hinting at their possible role as biomarkers of diagnosis and prognosis in thyroid cancer.
A recent study conducted by Maurei-Milan et al. investigated the link between lncRNAs ANRIL SNP, which plays the role of a tumor suppressor gene, and PTC, also investigating the link with various clinical features of this pathology. They determined ANRIL SNPs in rs11333048, rs4977574, rs4977574, rs1333040, and rs10757274 in 134 PTC patients and 155 controls. The results showed that the AAAC haplotype had a protective effect from PTC, whereas the CAAC and CAGT haplotypes were associated with increased cancer risk. Moreover, the rs1333048 CC variant was found more frequently in tumors >1 cm, whereas the rs4977574 AC variant was found predominantly in smaller tumor sizes. Furthermore, SNPs in rs10757274 and rs1333040 had a lower chance of advanced disease [84].
Chen et al. concluded that lncRNA RNA Polymerase II, I And III Subunit E (POLR2E) rs3787916 was associated with increased cancer risk [101]. Wen et al. investigated the association between MALAT1 SNP and thyroid cancer susceptibility and found that MALAT1 SNP rs619586 was a protective factor of PTC (p = 0.017) [102]. In 2022, Wang et al. published their results following an investigation between SNP rs8101923 within terminal differentiation-induced lncRNA (TINCR) and the risk of PTC and found that rs8101923 was a risk factor for the pathogenesis of PTC, with the G allele having a significantly associated higher risk of PTC [103].
There is still a very large spectrum for future research of lncRNAs SNPs that might have a great prognostic and/or therapeutic impact in DTC.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines11041075
References
- Sanabria, A.; Kowalski, L.P.; Shah, J.P.; Nixon, I.J.; Angelos, P.; Williams, M.D.; Rinaldo, A.; Ferlito, A. Growing incidence of thyroid carcinoma in recent years: Factors underlying overdiagnosis. Head Neck 2018, 40, 855–866.
- Shah, J.P. Thyroid carcinoma: Epidemiology, histology, and diagnosis. Clin. Adv. Hematol. Oncol. 2015, 13 (Suppl. S4), 3–6.
- Liu, Y.; Su, L.; Xiao, H. Review of Factors Related to the Thyroid Cancer Epidemic. Int. J. Endocrinol. 2017, 2017, 5308635.
- Orosco, R.K.; Hussain, T.; Brumund, K.T.; Oh, D.K.; Chang, D.C.; Bouvet, M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the Surveillance, Epidemiology, and End Results database. Thyroid 2015, 25, 125–132.
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40.
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2020, 67, 44–57.
- Melo, M.; da Rocha, A.G.; Batista, R.; Vingare, J.; Martins, M.J.; Costa, G.; Ribeiro, C.; Carrilho, F.; Leite, V.; Lobo, C.; et al. TERT, BRAF, and NRAS in Primary Thyroid Cancer and Metastatic Disease. J. Clin. Endocrinol. Metab. 2017, 102, 1898–1907.
- Vuong, H.G.; Altibi, A.M.A.; Duong, U.N.P.; Hassell, L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin. Endocrinol. 2017, 87, 411–417.
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182.
- Yang, W.; Zhang, T.; Song, X.; Dong, G.; Xu, L.; Jiang, F. SNP-Target Genes Interaction Perturbing the Cancer Risk in the Post-GWAS. Cancers 2022, 14, 5636.
- Takahashi, M.; Kawai, K.; Asai, N. Roles of the RET Proto-oncogene in Cancer and Development. JMA J. 2020, 3, 175–181.
- Santos, M.; Azevedo, T.; Martins, T.; Rodrigues, F.J.; Lemos, M.C. Association of RET genetic polymorphisms and haplotypes with papillary thyroid carcinoma in the Portuguese population: A case-control study. PLoS ONE 2014, 9, e109822.
- He, C.; Ma, J.; Jiang, Y.; Su, X.; Zhang, X.; Chen, W.; Ye, Z. Associations between RET tagSNPs and their haplotypes and susceptibility, clinical severity, and thyroid function in patients with differentiated thyroid cancer. PLoS ONE 2017, 12, e0187968.
- Ran, R.; Tu, G.; Li, H.; Wang, H.; Mou, E.; Liu, C. Genetic Variants Associated with Thyroid Cancer Risk: Comprehensive Research Synopsis, Meta-Analysis, and Cumulative Epidemiological Evidence. J. Oncol. 2021, 2021, 9967599.
- Trusolino, L.; Comoglio, P.M. Scatter-factor and semaphorin receptors: Cell signalling for invasive growth. Nat. Rev. Cancer 2002, 2, 289–300.
- Jung, K.H.; Park, B.H.; Hong, S.S. Progress in cancer therapy targeting c-Met signaling pathway. Arch. Pharm. Res. 2012, 35, 595–604.
- Ning, L.; Yu, Y.; Liu, X.; Ai, L.; Zhang, X.; Rao, W.; Shi, J.; Sun, H.; Yu, Q. Association Analysis of MET Gene Polymorphism with Papillary Thyroid Carcinoma in a Chinese Population. Int. J. Endocrinol. 2015, 2015, 405217.
- Uhliarova, B.; Hajtman, A. Hashimoto’s thyroiditis—An independent risk factor for papillary carcinoma. Braz. J. Otorhinolaryngol. 2018, 84, 729–735.
- Sun, L.; Wei, L.; Wei, L.; Li, D. Correlation between Bax gene polymorphisms and esophagus cancer. Oncol. Lett. 2018, 16, 7097–7101.
- Sahu, S.K.; Choudhuri, T. Lack of association between Bax promoter (-248G>A) single nucleotide polymorphism and susceptibility towards cancer: Evidence from a meta-analysis. PLoS ONE 2013, 8, e77534.
- Cardoso-Duarte, L.C.A.; Fratelli, C.F.; Pereira, A.S.R.; de Souza, J.N.G.; de Souza Freitas, R.; de Morais, R.M.; Sobrinho, A.B.; Silva, C.M.S.; de Oliveira, J.R.; de Oliveira, D.M.; et al. BAX gene (-248 G > A) polymorphism in a sample of patients diagnosed with thyroid cancer in the Federal District, Brazil. Int. J. Biol. Markers 2021, 36, 21–26.
- Pflaum, J.; Schlosser, S.; Müller, M. p53 Family and Cellular Stress Responses in Cancer. Front. Oncol. 2014, 4, 285.
- Menon, V.; Povirk, L. Involvement of p53 in the repair of DNA double strand breaks: Multifaceted Roles of p53 in homologous recombination repair (HRR) and non-homologous end joining (NHEJ). Subcell Biochem. 2014, 85, 321–336.
- Mauro, M.; Rego, M.A.; Boisvert, R.A.; Esashi, F.; Cavallo, F.; Jasin, M.; Howlett, N.G. p21 promotes error-free replication-coupled DNA double-strand break repair. Nucleic Acids Res. 2012, 40, 8348–8360.
- Furuta, T.; Hayward, R.L.; Meng, L.H.; Takemura, H.; Aune, G.J.; Bonner, W.M.; Aladjem, M.I.; Kohn, K.W.; Pommier, Y. p21CDKN1A allows the repair of replication-mediated DNA double-strand breaks induced by topoisomerase I and is inactivated by the checkpoint kinase inhibitor 7-hydroxystaurosporine. Oncogene 2006, 25, 2839–2849.
- Heidari, Z.; Harati-Sadegh, M.; Arian, A.; Maruei-Milan, R.; Salimi, S. The effect of TP53 and P21 gene polymorphisms on papillary thyroid carcinoma susceptibility and clinical/pathological features. IUBMB Life 2020, 72, 922–930.
- Lv, Z.; Kou, C.; Chen, N.; Jia, L.; Sun, X.; Gao, Y.; Bai, R.; Yang, M.; Cui, J. Single Nucleotide Polymorphisms in HOTAIR Are Related to Breast Cancer Risk and Prognosis in the Northeastern Chinese Population. Front. Oncol. 2021, 11, 706428.
- Kim, J.O.; Jun, H.H.; Kim, E.J.; Lee, J.Y.; Park, H.S.; Ryu, C.S.; Kim, S.; Oh, D.; Kim, J.W.; Kim, N.K. Genetic Variants of HOTAIR Associated With Colorectal Cancer Susceptibility and Mortality. Front. Oncol. 2020, 10, 72.
- Madadi Rad, R.; Pouladi, N.; Bosharani, N.N.; Alizadeh, A. Association between HOTAIR Rs1899663 G>T Gene Polymorphism and Thyroid Cancer Susceptibility. J. Babol. Univ. Med. Sci. 2022, 24, 95–102.
- Min, L.; Mu, X.; Tong, A.; Ling, C.; Yi, T.; Zhao, X. The association between HOTAIR polymorphisms and cancer susceptibility: An updated systemic review and meta-analysis. Onco. Targets Ther. 2018, 11, 791–800.
- Çağlayan, M.; Wilson, S.H. Oxidant and environmental toxicant-induced effects compromise DNA ligation during base excision DNA repair. DNA Repair 2015, 35, 85–89.
- Zhao, J.Z.; Tan, X.R.; Zhao, M.; Mao, X.C.; Jiang, L. Association between the X-ray repair cross-complementing group 1 Arg194Trp polymorphism and thyroid carcinoma susceptibility: A meta-analysis. Genet. Mol. Res. 2016, 15.
- Liu, S.Y.; Xue, W. XRCC1 Arg194Trp polymorphism and thyroid cancer. J. Endocrinol. Invest. 2020, 43, 749–753.
- Bains, A.; Mur, T.; Wallace, N.; Noordzij, J.P. The Role of Vitamin D as a Prognostic Marker in Papillary Thyroid Cancer. Cancers 2021, 13, 3516.
- Zhao, J.; Wang, H.; Zhang, Z.; Zhou, X.; Yao, J.; Zhang, R.; Liao, L.; Dong, J. Vitamin D deficiency as a risk factor for thyroid cancer: A meta-analysis of case-control studies. Nutrition 2019, 57, 5–11.
- Kim, J.R.; Kim, B.H.; Kim, S.M.; Oh, M.Y.; Kim, W.J.; Jeon, Y.K.; Kim, S.S.; Lee, B.L.; Kim, Y.K.; Kim, I.J. Low serum 25 hydroxyvitamin D is associated with poor clinicopathologic characteristics in female patients with papillary thyroid cancer. Thyroid 2014, 24, 1618–1624.
- Liu, Y.; Li, C.; Chen, P.; Li, X.; Li, M.; Guo, H.; Li, J.; Chu, R.; Wang, H. Polymorphisms in the vitamin D Receptor (VDR) and the risk of ovarian cancer: A meta-analysis. PLoS ONE 2013, 8, e66716.
- Pan, Z.; Chen, M.; Hu, X.; Wang, H.; Yang, J.; Zhang, C.; Pan, F.; Sun, G. Associations between VDR gene polymorphisms and colorectal cancer susceptibility: An updated meta-analysis based on 39 case-control studies. Oncotarget 2018, 9, 13068–13076.
- Iqbal, M.U.N.; Khan, T.A. Association between Vitamin D receptor (Cdx2, Fok1, Bsm1, Apa1, Bgl1, Taq1, and Poly (A)) gene polymorphism and breast cancer: A systematic review and meta-analysis. Tumour Biol. 2017, 39, 1010428317731280.
- Beysel, S.; Eyerci, N.; Pinarli, F.A.; Apaydin, M.; Kizilgul, M.; Caliskan, M.; Ozcelik, O.; Kan, S.; Cakal, E. VDR gene FokI polymorphism as a poor prognostic factor for papillary thyroid cancer. Tumour Biol. 2018, 40, 1010428318811766.
- Cocolos, A.M.; Muresan, A.; Caragheorgheopol, A.; Ghemigian, M.; Ioachim, D.; Poiana, C. Vitamin D Status and VDR Polymorphisms as Prognostic Factors in Differentiated Thyroid Carcinoma. In Vivo 2022, 36, 2434–2441.
- Chiodoni, C.; Colombo, M.P.; Sangaletti, S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010, 29, 295–307.
- Tu, Y.; Chen, C.; Fan, G. Association between the expression of secreted phosphoprotein—Related genes and prognosis of human cancer. BMC Cancer 2019, 19, 1230.
- Wang, B.; Chen, K.; Xu, W.; Chen, D.; Tang, W.; Xia, T.S. Integrative genomic analyses of secreted protein acidic and rich in cysteine and its role in cancer prediction. Mol. Med. Rep. 2014, 10, 1461–1468.
- Su, X.; Xu, B.-H.; Zhou, D.-L.; Ye, Z.-L.; He, H.-C.; Yang, X.-H.; Zhang, X.; Liu, Q.; Ma, J.-J.; Shao, Q.; et al. Polymorphisms in matricellular SPP1 and SPARC contribute to susceptibility to papillary thyroid cancer. Genomics 2020, 112, 4959–4967.
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768.
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260, Erratum in Eur. J. Med. Chem. 2020, 205, 112642.
- Farina, A.R.; Mackay, A.R. Gelatinase B/MMP-9 in Tumour Pathogenesis and Progression. Cancers 2014, 6, 240–296.
- Dobrescu, R.; Picu, C.; Caragheorgheopol, A.; Danda, D.; Ioachim, D.; Goldstein, A.; Badiu, C. Serum Matrix metalloproteinase-9 (MMP-9) can help identify patients with papillary thyroid cancer at high risk of persistent disease: Value and limitations of a potential marker of neoplasia. Cancer Biomark. 2020, 29, 337–346.
- Dobrescu, R.; Schipor, S.; Manda, D.; Caragheorgheopol, A.; Badiu, C. Matrix metalloproteinase-9 (MMP-9) promoter -1562C/T functional polymorphism is associated with an increased risk to develop micropapillary thyroid carcinoma. Cancer Biomark. 2022, 34, 555–562.
- He, H.; Li, W.; Liyanarachchi, S.; Wang, Y.; Yu, L.; Genutis, L.K.; Maharry, S.; Phay, J.E.; Shen, R.; Brock, P.; et al. The Role of NRG1 in the Predisposition to Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2018, 103, 1369–1379.
- Wang, Y.-L.; Feng, S.-H.; Guo, S.-C.; Wei, W.-J.; Li, D.-S.; Wang, Y.; Wang, X.; Wang, Z.-Y.; Ma, Y.-Y.; Jin, L.; et al. Confirmation of papillary thyroid cancer susceptibility loci identified by genome-wide association studies of chromosomes 14q13, 9q22, 2q35 and 8p12 in a Chinese population. J. Med. Genet. 2013, 50, 689–695.
- Gudmundsson, J.; Sulem, P.; Gudbjartsson, D.F.; Jonasson, J.G.; Masson, G.; He, H.; Jonasdottir, A.; Sigurdsson, A.; Stacey, S.A.; Johannsdottir, H.; et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nat. Genet. 2012, 44, 319–322.
- Guo, Y.; Zhang, W.; He, R.; Zheng, C.; Liu, X.; Ge, M.; Xu, J. Investigating the Association Between rs2439302 Polymorphism and Thyroid Cancer: A Systematic Review and Meta-Analysis. Front. Surg. 2022, 9, 877206.
- Credendino, S.C.; Moccia, C.; Amendola, E.; D’Avino, G.; Di Guida, L.; Clery, E.; Greco, A.; Bellevicine, C.; Brunetti, A.; De Felice, M.; et al. FOXE1 Gene Dosage Affects Thyroid Cancer Histology and Differentiation In Vivo. Int. J. Mol. Sci. 2020, 22, 25.
- Penna-Martinez, M.; Epp, F.; Kahles, H.; Ramos-Lopez, E.; Hinsch, N.; Hansmann, M.-L.; Selkinski, I.; Grünwald, F.; Holzer, K.; Bechstein, W.O.; et al. FOXE1 association with differentiated thyroid cancer and its progression. Thyroid 2014, 24, 845–851.
- Chen, Y.H.; Zhang, Y.Q. Exploration of the association between FOXE1 gene polymorphism and differentiated thyroid cancer: A meta-analysis. BMC Med. Genet. 2018, 19, 83.
- Mehrazin, A.; Safarpour, H.; Davoudi, S.T.; Parsamanesh, N.; Saeedi, F.; Miri-Moghaddam, E. Network-Based Analysis Reveals Association of FOXE1 Gene Polymorphisms in Thyroid Cancer Patients; A Case-Control Study in Southeast of Iran. Asian Pac. J. Cancer Prev. 2020, 21, 2771–2776.
- Carvalho, D.P.; Dupuy, C. Thyroid hormone biosynthesis and release. Mol. Cell Endocrinol. 2017, 458, 6–15.
- Sasaki, S.; Matsushita, A.; Kuroda, G.; Nakamura, H.M.; Oki, Y.; Suda, T. The mechanism of negative transcriptional regulation by thyroid hormone: Lessons from the thyrotropin β subunit gene. Vitam. Horm. 2018, 106, 97–127.
- Partsch, C.J.; Riepe, F.G.; Krone, N.; Sippell, W.G.; Pohlenz, J. Initially elevated TSH and congenital central hypothyroidism due to a homozygous mutation of the TSH beta subunit gene: Case report and review of the literature. Exp. Clin. Endocrinol. Diabetes 2006, 114, 227–234.
- Vuissoz, J.M.; Deladoëy, J.; Buyukgebiz, A.; Cemeroglu, P.; Gex, G.; Gallati, S.; Mullis, P.E. New autosomal recessive mutation of the TSH-beta subunit gene causing central isolated hypothyroidism. J. Clin. Endocrinol. Metab. 2001, 86, 4468–4471.
- AlRasheed, M.M. TSH-β gene polymorphism in Saudi patients with thyroid cancer: A case-control study. Saudi Pharm. J. 2022, 30, 1538–1542.
- Zara-Lopes, T.; Galbiatti-Dias, A.L.S.; Castanhole-Nunes, M.M.U.; Padovani-Júnior, J.A.; Maniglia, J.V.; Pavarino, E.C.; Goloni-Bertello, E.M. Polymorphisms in MTHFR, MTR, RFC1 and CßS genes involved in folate metabolism and thyroid cancer: A case-control study. Arch. Med. Sci. 2019, 15, 522–530.
- Alshatwi, A.A. Breast cancer risk, dietary intake, and methylenetetrahydrofolate reductase (MTHFR)single nucleotide polymorphisms. Food Chem. Toxicol. 2018, 48, 1881–1885, Erratum in Food Chem. Toxicol. 2018, 118, 973.
- Weiner, A.S.; Boyarskikh, U.A.; Voronina, E.N.; Voronina, E.N.; Selezneva, I.A.; Sinkina, T.V.; Lazarev, A.F.; Petrova, V.D.; Filipenko, M.L. Polymorphisms in the folate-metabolizing genes MTR, MTRR, and CBS and breast cancer risk. Cancer Epidemiol. 2012, 36, e95–e100.
- Zhuo, X.; Song, J.; Li, D.; Wu, Y.; Zhou, Q. MTHFR C677T polymorphism interaction with heavy alcohol consumption increases head and neck carcinoma risk. Sci. Rep. 2015, 5, 10671.
- Prasad, V.V.; Wilkhoo, H. Association of the functional polymorphism C677T in the methylenetetrahydrofolate reductase gene with colorectal, thyroid, breast, ovarian, and cervical cancers. Onkologie 2011, 34, 422–426.
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499.
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259.
- Lumachi, F.; Basso, S.M.; Orlando, R. Cytokines, thyroid diseases and thyroid cancer. Cytokine 2010, 50, 229–233.
- Zhu, Z.; Liu, J.B.; Liu, X.; Qian, L. Association of interleukin 10 rs1800896 polymorphism with susceptibility to breast cancer: A meta-analysis. J. Int. Med. Res. 2020, 48, 300060520904863.
- Çil, E.; Kumral, A.; Kanmaz-Özer, M.; Vural, P.; Doğru-Abbasoğlu, S.; Altuntaş, Y.; Uysal, M. Interleukin-10-1082 gene polymorphism is associated with papillary thyroid cancer. Mol. Biol. Rep. 2014, 41, 3091–3097.
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408.
- Park, S.W.; Kim, M.K.; Kwon, K.H.; Kim, J. Association between a promoter polymorphism (rs2192752, -1028A/C) of interleukin 1 receptor, type I (IL1R1) and location of papillary thyroid carcinoma in a Korean population. Int. J. Immunogenet. 2012, 39, 501–507.
- Xie, M.; Zhang, D.; Zhang, Y.; Yang, X.; Su, Y.; Wang, Y.; Huang, H.; Han, H.; Li, W.; Fu, K.; et al. Association of genetic polymorphisms in IL-1R1 and IL-1R2 genes with IgA nephropathy in the Han Chinese population. Oncotarget 2017, 8, 50673–50679.
- Na, Y.; Bai, R.; Zhao, Z.; Wei, Y.; Li, D.; Wang, Y.; Sun, C.; Sun, L.; Zhang, B.; Jin, T.; et al. IL1R1 gene polymorphisms are associated with knee osteoarthritis risk in the Chinese Han population. Oncotarget 2017, 8, 4228–4233.
- Vambutas, A.; DeVoti, J.; Goldofsky, E.; Gordon, M.; Lesser, M.; Bonagura, V. Alternate splicing of interleukin-1 receptor type II (IL1R2) in vitro correlates with clinical glucocorticoid responsiveness in patients with AIED. PLoS ONE 2009, 4, e5293.
- Xiong, Z.; Sun, Y.; Wu, J.; Niu, F.; Jin, T.; Li, B. Genetic polymorphisms in IL1R1 and IL1R2 are associated with susceptibility to thyroid cancer in the Chinese Han population. J. Gene Med. 2019, 21, e3093.
- Li, H.; Duan, N.; Zhang, Q.; Shao, Y. IL1A & IL1B genetic polymorphisms are risk factors for thyroid cancer in a Chinese Han population. Int. Immunopharmacol. 2019, 76, 105869.
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, C254–C262.
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The Double-Faced Role of Nitric Oxide and Reactive Oxygen Species in Solid Tumors. Antioxidants 2020, 9, 374.
- Cerqueira, A.R.D.; Fratelli, C.F.; Duarte, L.C.A.C.; Pereira, A.S.R.; de Morais, R.M.; Sobrinho, A.B.; de Souza Silva, C.M.; da Silva, I.C.R.; de Oliveira, J.R. The impact of NOS3 gene polymorphism on papillary thyroid cancer susceptibility in patients undergoing radioiodine therapy. Int. J. Biol. Markers 2020, 35, 87–91.
- Maruei-Milan, R.; Heidari, Z.; Aryan, A.; Asadi-Tarani, M.; Salimi, S. Long non-coding RNA ANRIL polymorphisms in papillary thyroid cancer and its severity. Br. J. Biomed. Sci. 2021, 78, 58–62.
- Tano, K.; Akimitsu, N. Long non-coding RNAs in cancer progression. Front. Genet. 2012, 3, 219.
- Tornesello, M.L.; Faraonio, R.; Buonaguro, L.; Annunziata, C.; Starita, N.; Cerasuolo, A.; Pezzuto, F.; Tornesello, A.L.; Buonaguro, F.M. The Role of microRNAs, Long Non-coding RNAs, and Circular RNAs in Cervical Cancer. Front. Oncol. 2020, 10, 150.
- Khan, R.; Shaheen, H.; Mansoor, Q.; Abbasi, S.A.; Fatima, S.; Ammar, A.; Baig, R.M. Genetic predisposition of SNPs in miRNA-149 (rs2292832) and FOXE1 (rs3758249) in thyroid Cancer. Mol. Biol. Rep. 2021, 48, 7801–7809.
- Khan, R.; Abbasi, S.A.; Mansoor, Q.; Ahmed, M.N.; Mir, K.B.; Baig, R.M. Analysis of Rare Alleles of miRNA-146a (rs2910164) and miRNA-34b/c (rs4938723) as a Prognostic Marker in Thyroid Cancer in Pakistani Population. Diagnostics 2022, 12, 2495.
- Yang, M.; Tian, J.; Guo, X.; Yang, Y.; Guan, R.; Qiu, M.; Li, Y.; Sun, X.; Zhen, Y.; Zhang, Y.; et al. Long noncoding RNA are aberrantly expressed in human papillary thyroid carcinoma. Oncol. Lett. 2016, 12, 544–552.
- Peng, X.; Zhang, K.; Ma, L.; Xu, J.; Chang, W. The Role of Long Non-Coding RNAs in Thyroid Cancer. Front. Oncol. 2020, 10, 941.
- Zhu, H.; Lv, Z.; An, C.; Shi, M.; Pan, W.; Zhou, L.; Yang, W.; Yang, M. Onco-lncRNA HOTAIR and its functional genetic variants in papillary thyroid carcinoma. Sci. Rep. 2016, 6, 31969.
- Zhao, J.-J.; Hao, S.; Wang, L.-L.; Hu, C.-Y.; Zhang, S.; Guo, L.-J.; Zhang, G.; Gao, B.; Jiang, Y.; Tian, W.-G.; et al. Long non-coding RNA ANRIL promotes the invasion and metastasis of thyroid cancer cells through TGF-β/Smad signaling pathway. Oncotarget 2016, 7, 57903–57918.
- Jendrzejewski, J.; He, H.; Radomska, H.S.; Li, W.; Tomsic, J.; Liyanarachchi, S.; Davuluri, R.V.; Nagy, R.; de la Chapelle, A. The polymorphism rs944289 predisposes to papillary thyroid carcinoma through a large intergenic noncoding RNA gene of tumor suppressor type. Proc. Natl. Acad. Sci. USA 2012, 109, 8646–8651.
- Jendrzejewski, J.; Thomas, A.; Liyanarachchi, S.; Eiterman, A.; Tomsic, J.; He, H.; Radomska, H.S.; Li, W.; Nagy, R.; Sworczak, K.; et al. PTCSC3 Is Involved in Papillary Thyroid Carcinoma Development by Modulating S100A4 Gene Expression. J. Clin. Endocrinol. Metab. 2015, 100, E1370–E1377.
- Liao, T.; Qu, N.; Shi, R.L.; Guo, K.; Ma, B.; Cao, Y.-M.; Xiang, J.; Lu, Z.-W.; Zhu, Y.-X.; Li, D.-S.; et al. BRAF-activated LncRNA functions as a tumor suppressor in papillary thyroid cancer. Oncotarget 2017, 8, 238–247.
- Wang, C.; Yan, G.; Zhang, Y.; Jia, X.; Bu, P. Long non-coding RNA MEG3 suppresses migration and invasion of thyroid carcinoma by targeting of Rac1. Neoplasma 2015, 62, 541–549.
- Huang, J.-K.; Ma, L.; Song, W.-H.; Lu, B.-Y.; Huang, Y.-B.; Dong, H.-M.; Ma, X.-K.; Zhu, Z.-Z.; Zhou, R. LncRNA-MALAT1 Promotes Angiogenesis of Thyroid Cancer by Modulating Tumor-Associated Macrophage FGF2 Protein Secretion. J. Cell Biochem. 2017, 118, 4821–4830.
- Li, T.; Yang, X.-D.; Ye, C.-X.; Shen, Z.-Y.; Yang, Y.; Wang, B.; Guo, P.; Gao, Z.-D.; Ye, Y.-J.; Jiang, K.-W.; et al. Long noncoding RNA HIT000218960 promotes papillary thyroid cancer oncogenesis and tumor progression by upregulating the expression of high mobility group AT-hook 2 (HMGA2) gene. Cell Cycle 2017, 16, 224–231.
- Huang, F.; Zhang, Q.; Chen, W.; Zhang, H.; Lu, G.; Chen, J.; Qiu, C. Long noncoding RNA cancer susceptibility candidate 2 suppresses papillary thyroid carcinoma growth by inactivating the AKT/ERK1/2 signaling pathway. J. Cell Biochem. 2019, 120, 10380–10390.
- Chen, C.; Zhou, L.; Wang, H.; Chen, J.; Li, W.; Liu, W.; Shen, M.; Liu, H.; Fu, X. Long noncoding RNA CNALPTC1 promotes cell proliferation and migration of papillary thyroid cancer via sponging miR-30 family. Am. J. Cancer Res. 2018, 8, 192–206.
- Chen, B.; Li, J.; Yi, C.; Jiao, Y.; Gu, X.; Feng, X. Long non-coding RNA POLR2E rs3787016 is associated with the risk of papillary thyroid carcinoma in Chinese population. Pathol. Res. Pract. 2018, 214, 1040–1044.
- Wen, J.; Chen, L.; Tian, H.; Li, J.; Zhang, M.; Cao, Q.; Zhang, W.; Chen, S.; Shi, L. Effect of MALAT1 Polymorphisms on Papillary Thyroid Cancer in a Chinese Population. J. Cancer 2019, 10, 5714–5721.
- Wang, Q.; Huang, H.; Chen, P.; Xiao, X.; Luo, X.; Wang, Y.; Long, S.; Gao, L.; Zhang, L. Genetic predisposition to papillary thyroid carcinoma is mediated by a long non-coding RNA TINCR enhancer polymorphism. Int. Immunopharmacol. 2022, 109, 108796.
This entry is offline, you can click here to edit this entry!
