The fetal environment is modulated by the placenta, which integrates and transduces information from the maternal environment to the fetal developmental program and adapts rapidly to changes through epigenetic mechanisms that respond to internal (hereditary) and external (environmental and social) signals. Consequently, the fetus corrects the trajectory of own development. During the last trimester of gestation, plasticity shapes the fetal brain, and prematurity can alter the typical developmental trajectories. Prenatal music stimulation had positive effects on fetus, newborn, and pregnant mother while post-natal exposure affected the neurodevelopment of the preterm infants and parental interaction.
- epigenetic
- fetal development
- prenatal maternal stress
- music
- premature newborns
1. Introduction
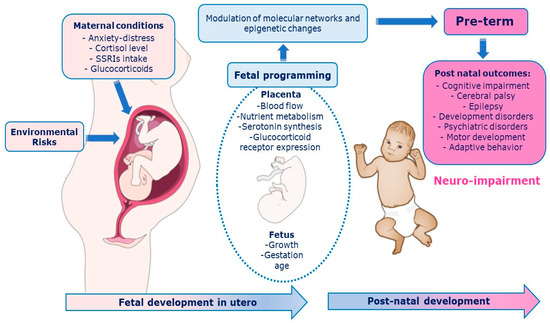
2. Musical Stimulation on Placental Programming of Preterm Infants
References
- Glynn, L. M.; Sandman, C. A. Prenatal origins of neurological development: a critical period for fetus and mother. Dir. Psychol. Sci. 2011, 20 (6), 384-389. https://www.jstor.org/stable/23213078#metadata_info_tab_contents.
- Barker, D. J. Fetal origins of coronary heart disease. BMJ 1995, 311 (6998), 171-174. doi:10.1136/bmj.311.6998.171.
- Spencer, H.G.; Pleasants, A.B.; Gluckman, P.D.; Wake, G.C. A model of optimal timing for a predictive adaptive response. Dev. Orig. Health Dis. 2022, 13 (1), 101-107. doi: 10.1017/S2040174420001361.
- Gluckman, P. D.; Hanson, M. A.¸Low, F. M. The role of developmental plasticity and epigenetics in human health. Birth Defects Res. C Embryo Today, 2011, 93 (1), 12-18. doi:10.1002/bdrc.20198.
- Gluckman, P. D.; Hanson, M. A. Living with the past: Evolution, development, and patterns of disease. Science, 2004, 305 (5691), 1733-1736. doi:10.1126/science.1095292.
- Sandman, C. A.; Davis, E. P.; Buss, C.; Glynn, L. M. Prenatal programming of human neurological function. J. Pept. 2011, 2011. doi:10.1155/2011/837596.
- Glover, V. Annual research review: Prenatal stress and the origins of psychopathology: An evolutionary perspective. Child Psychol. Psychiatry, 2011, 52 (4), 356-367. doi:10.1111/j.1469-7610.2011.02371.x.
- Paquette, A. G.; Lesseur, C.; Armstrong, D. A.; Koestler, D. C.; Appleton, A. A.; Lester, B. M.; Marsit, C. J. Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics, 2013, 8 (8), 796-801. doi:10.4161/epi.25358.
- Challis, J. R. G.; Sloboda, D.; Matthews, S. G.; Holloway, A.; Alfaidy, N.; Patel, F. A.; Newnham, J. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Cell. Endocrinol. 2001, 185 (1-2), 135-144. doi:10.1016/s0303-7207(01)00624-4.
- Feldman, R. From biological rhythms to social rhythms: Physiological precursors of mother-infant synchrony. Psychol. 2006, 42 (1), 175-188. doi:10.1037/0012-1649.42.1.175
- Thompson, L. A.; Trevathan, W.R. Cortisol reactivity, maternal sensitivity, and learning in 3-month-old infants. Infant Behav. Dev. 2008, 31 (1), 92-106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2277326/.
- Bonnin, A.; Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neurosci. 2011, 197, 1-7. doi:10.1016/j.neuroscience.2011.10.005
- Sonier, B., Lavigne, C., Arseneault, M., Ouellette, R., & Vaillancourt, C. Expression of the 5-HT2A serotoninergic receptor in human placenta and choriocarcinoma cells: Mitogenic implications of serotonin. Placenta 2005, 26(6), 484-490. doi:10.1016/j.placenta.2004.08.003.
- Homberg, J. R.; Contet, C. Deciphering the interaction of the corticotropin-releasing factor and serotonin brain systems in anxiety-related disorders. Neurosci. 2009, 29 (44), 13743-13745. doi:10.1523/JNEUROSCI.4362-09.2009.
- Velasquez, J. C.; Goeden, N.; Bonnin, A. Placental serotonin: Implications for the developmental effects of SSRIs and maternal depression. Cell. Neurosci. 2013, 7, 47. https://doi.org/10.3389/fncel.2013.00047.
- Shallie, P.D.; Naicker, T. The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. J. Dev. Neurosci. 2019, 73, 41-49.doi: 10.1016/j.ijdevneu.2019.01.003.
- Nugent, B.M.; Bale, T.L. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 2015, 39, 28-37. doi: 10.1016/j.yfrne.2015.09.001.
- Kisilevsky, B. S.; Hains, S. M. J.; Jacquet, A. Y.; Granier‐Deferre, C.; Lecanuet, J. P.. Maturation of fetal responses to music. Sci., 2004, 7 (5), 550-559. doi:10.1111/j.1467-7687.2004.00379.x.
- He, H.; Huang, J.; Zhao, X.; Li, Z. The effect of prenatal music therapy on fetal and neonatal status: A systematic review and meta-analysis. Ther. Med. 2021, 60, 102756. doi:10.1016/j.ctim.2021.102756.
- Pino, O. Fetal memory: The effects of prenatal auditory experience on human development. BAOJ Med Nursing, 2016, 2 (4), 2. doi:10.24947/BAOJMN/2/2/120.
- y Cajal, C. L. R. Antenatal study of the Heschl’s gyrus: The first step to understanding prenatal learning. Hypotheses, 2019, 130, 109290. doi:10.1016/j.mehy.2019.109290.
- Lu, J.; Cui, Y.; Cai, R.; Mao, Y.; Zhang, J.; Sun, X. Early auditory deprivation alters expression of NMDA receptor subunit NR1 mRNA in the rat auditory cortex. Neurosci. Res. 2008, 86 (6), 1290-1296. doi:10.1002/jnr.21577.
- McMahon, E.; Wintermark, P.; Lahav, A. Auditory brain development in premature infants: The importance of early experience. Ann N Y Acad Sci. 2012, 1252 (1), 17-24. doi:10.1111/j.1749-6632.2012.06445.x.
- Filippa, M.; Lordier, L.; De Almeida, J.S.; Monaci, M.G.; Adam-Darque, A.;Grandjean, D.; Kuhn, P.; Hüppi, P.S. Early vocal contact and music in the NICU: New insights into preventive interventions. Pediatr Res. 2020, 87, 249-264 (2020). https://doi.org/10.1038/s41390-019-0490-9.
- Caskey, M.; Vohr B. Assessing language and language environment of high-risk infants and children: A new approach. Acta Paediatr. 2013, 102, 451-461. doi: 10.1111/apa.12195.
- Richter, J.; Ostovar, R. “It don’t mean a thing if it ain’t got that swing” - An alternative concept for understanding the evolution of dance and music in human beings. Human Neurosci. 2016, 10, 1-13. doi: 10.3389/fnhum.2016.00485.
- Kellam, B.; Bhatia, J. Sound spectral analysis in the intensive care nursery: measuring high-frequency sound. J Pediatric Nursing, 2008, 23 (4), 317-323. doi:10.1016/j.pedn.2007.09.009.
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the human cerebral cortex: Boulder Committee revisited. Nature Rev Neurosci. 2008, 9(2), 110-122. https://doi.org/10.1038/nrn2252.
- deRegnier, R. A.; Wewerka, S.; Georgieff, M. K.; Mattia, F.; Nelson, C. A. Influences of postconceptional age and postnatal experience on the development of auditory recognition memory in the newborn infant. Psychobiol. 2002, 41 (3), 216-225. doi:10.1002/dev.10070.
- Anderson, P.; Doyle, L. W. Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003, 289 (24), 3264-3272. doi:10.1001/jama.289.24.3264.
- Bhutta, A. T.; Cleves, M. A.; Casey, P. H.; Cradock, M. M; Anand, K. J. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA, 2002, 288 (6), 728-737. doi:10.1001/jama.288.6.728.
- Montagna, A.; Nosarti, C. Socio-emotional development following very preterm birth: Pathways to psychopathology. Psychol. 2016, 7, 80. doi:10.3389/fpsyg.2016.00080.
- Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Res. 2011, 69 (5), 11R-8R. doi: 10.1203/PDR.0b013e318212faa0.
- Nosarti, C.; Reichenberg, A.; Murray, R. M.; Cnattingius, S.; Lambe, M. P.; Yin, L.; Hultman, C. M. Preterm birth and psychiatric disorders in young adult life. Gen. Psychiatry, 2012, 69 (6), 610-617. doi:10.1001/archgenpsychiatry.2011.1374.
- Treyvaud, K.; Ure, A.; Doyle, L. W.; Lee, K. J.; Rogers, C. E.; Kidokoro, H.; Anderson, P. J. Psychiatric outcomes at age seven for very preterm children: Rates and predictors. Child Psychol. Psychiatry, 2013, 54(7), 772-779. doi:10.1111/jcpp.12040.
- Salimpoor, V. N.; Benovoy, M.; Longo, G.; Cooperstock, J. R.; Zatorre, R. J. The rewarding aspects of music listening are related to degree of emotional arousal. PloS One 2009, 4 (10), e7487. https://doi.org/10.1371/journal.pone.0007487.
- Janata, P; Birk, J. L.; Van Horn, J. D.; Leman, M.; Tillmann, B.; Bharucha, J. J. The cortical topography of tonal structures underlying Western music. Science 2002, 298 (5601), 2167-2170. doi:10.1126/science.1076262.
- Koelsch, S. Towards a neural basis of music-evoked emotions. Trends Cogn. Sci. 2010, 14 (3), 131-137. doi:10.1016/j.tics.2010.01.002.
- Koelsch, S. Brain correlates of music-evoked emotions. Rev. Neurosci. 2014, 15 (3), 170-180. https://doi.org/10.1038/nrn3666.
- Blood, A. J.; Zatorre, R. J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Natl. Acad. Sci. USA 2001, 98 (20), 11818-11823. https://doi.org/10.1073/pnas.19135589.
- Brown, S.; Martinez, M. J.; Parsons, L. M. Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport 2004, 15(13), 2033-2037. doi:10.1097/00001756-200409150-00008.
- Anderson, D. E.; Patel A. D. Infants born preterm, stress, and neurodevelopment in the neonatal intensive care unit: Might music have an impact? Med. Child. Neurol. 2018, 60 (3), 256-266. doi:10.1111/dmcn.13663.
- van der Heijden, M. J.; Oliai Araghi, S.; Jeekel, J.; Reiss, I. K. M.; Hunink, M. M.; Van Dijk, M. Do hospitalized premature infants benefit from music interventions? A systematic review of randomized controlled trials. PloS One 2016, 11 (9), e0161848. doi:10.1371/journal.pone.0161848.
- Chaudhury, S.; Nag, T. C.; Jain, S.; Wadhwa, S. Role of sound stimulation in reprogramming brain connectivity. Biosci. 2013,38 (3), 605-614. doi:10.1007/s12038-013-9341-8
- Chang, S. C.; Chen, C. H. Effects of music therapy on women’s physiologic measures, anxiety, and satisfaction during cesarean delivery. Nurs. Health 2005, 28 (6), 453-461. doi:10.1002/nur.20102.
- Perani, D.; Saccuman, M. C.; Scifo, P.; Spada, D.; Andreolli, G.; Rovelli, R.; Koelsch, S. Functional specializations for music processing in the human newborn brain. Natl. Acad. Sci. USA 2010, 107 (10), 4758-4763. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2842045/.
- Sanes, D. H.; Woolley, S. M. A behavioral framework to guide research on central auditory development and plasticity. Neuron. 2011, 72 (6), 912-929. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3244881/.
- Skoe, E.; Chandrasekaran, B. The layering of auditory experiences in driving experience-dependent subcortical plasticity. Hear Res. 2014, 311, 36-48. doi:10.1016/j.heares.2014.01.002.
- Moher, D.; Liberati, R.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009, 6(7), e1000097 https://doi: 10.1371/journal.pmed.1000097.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A. et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372 (71) doi: https://doi.org/10.1136/bmj.n71.
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J. et al. Cochrane handbook for systematic reviews of interventions version 6. Cochrane Training, 2020. Available online: https://www.training.cochrane.org/handbook (accessed June 2022).
- Arya, R.; Chansoria, M.; Konanki, R.; Tiwari, D. K. Maternal music exposure during pregnancy influences neonatal behaviour: An open-label randomized controlled trial. J. Pediatr., 2012, 901812. doi:10.1155/2012/901812.
- García González, J.; Ventura Miranda, M.I.; Manchon García, F.; Gascón, M. M.; Mullor, M. R.; Carreño, T. P. Effects of prenatal music stimulation on fetal cardiac state, newborn anthropometric measurements, and vital signs of pregnant women: A randomized controlled trial. Ther. Clin. Pract. 2017, 27, 61-67. doi:10.1016/j.ctcp.2017.03.004.
- Haslbeck, F.B.; Jakab, A.; Held, U.; Bassler, D.; Bucher, H.U.; Hagmann C. Creative music therapy to promote brain function and brain structure in preterm infants: A randomized controlled pilot study. Neuroimage Clin. 2020, 25, 102171. doi: 10.1016/j.nicl.2020.102171.
- Lejeune, F.; Lordier, L.; Pittet, M. P.; Schoenhals, L.; Grandjean, D.; Hüppi, P. S; Borradori Tolsa, C. Effects of an early postnatal music intervention on cognitive and emotional development in preterm children at 12 and 24 months: Preliminary findings. Psychol. 2019, 10, 494. https://doi.org/10.3389/fpsyg.2019.00494.
- Lordier, L.; Loukas, S.; Grouiller, F.; Vollenweider, A.; Vasung, L.; Meskaldij, D. E.; Grandjean, D. Music processing in preterm and full-term newborns: A psychophysiological interaction (PPI) approach in neonatal fMRI. Neuroimage, 2019, 185, 857-864. doi:10.1016/j.neuroimage.2018.03.078.
- Lordier, L.; Meskaldji, D. E.; Grouiller, F.; Pittet, M. P.; Vollenweider, A.; Vasung, L.; Hüppi, P. S. Music in premature infants enhances high-level cognitive brain networks. Natl. Acad. Sci. USA 2019,116 (24), 12103-12108. https://doi.org/10.1073/pnas.181753611.
- Arnon, S.; Epstein, S.; Ghetti, C.; Bauer-Rusek, S.; Taitelbaum-Swead, R.; Yakobson, D. Music therapy intervention in an open bay Neonatal Intensive Care Unit room is associated with less noise and higher signal to noise ratios: A case-control study. Children 2022, 9 (8), 1187. doi: 10.3390/children9081187.
- Kehl S.M.; La Marca-Ghaemmaghami P.; Haller M.; Pichler-Stachl E.; Bucher H.U.; Bassler D.; Haslbeck F.B. Creative music therapy with premature infants and their parents: A mixed-method pilot study on parents’ anxiety, stress and depressive symptoms and parent-Infant attachment. Int. J. Environ. Res. Public Health, 2021, 18 (1), 265. doi: 10.3390/ijerph18010265.
- Webb, A. R.; Heller, H. T.; Benson, C. B.; Lahav, A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Procl. Natl. Acad. Sci. USA, 2015, 112 (10), 3152-3157. https://doi.org/10.1073/pnas.14149241.
- Granier-Deferre, C.; Bassereau, S.; Ribeiro, A.; Jacquet, A. Y.; DeCasper, A. J. A melodic contour repeatedly experienced by human near-term fetuses elicits a profound cardiac reaction one month after birth. PLoS One 2011, 6 (2), e17304. https://doi.org/10.1371/journal.pone.0017304.
- López-Teijón, M.; García-Faura, Á.; Prats-Galino, A. Fetal facial expression in response to intravaginal music emission. Ultrasound 2015, 23(4), 216-223. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4616906/.
- Partanen, E.; Kujala, T.; Tervaniemi, M.; Huotilainen, M. Prenatal music exposure induces long-term neural effects. PloS One 2013, 8 (10), e78946. https://doi.org/10.1371/journal.pone.0078946.
- Partanen, E.; Kujala, T.; Näätänen, R.; Liitola, A.; Sambeth, A.; Huotilainen, M. Learning-induced neural plasticity of speech processing before birth. Proc. Natl. Acad. Sci. USA 2013, 110 (37), 15145-15150. https://doi.org/10.1073/pnas.13021591.
- Buss, C.; Davis, E. P.; Hobel, C. J.; Sandman, C. A. Maternal pregnancy-specific anxiety is associated with child executive function at 6-9 years age. Stress 2011, 14(6), 665-676. doi:10.3109/10253890.2011.623250.
- Kim, D. J.; Davis, E. P.; Sandman, C. A.; Sporns, O.; O’Donnell, B. F.; Buss, C.; Hetrick, W. P. Prenatal maternal cortisol has sex-specific associations with child brain network properties. Cortex 2017, 27 (11), 5230-5241. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6084613/.
- Lin, Y.; Xu, J.; Huang, J.; Jia, Y.; Zhang, J.; Yan, C.; Zhang, J. Effects of prenatal and postnatal maternal emotional stress on toddlers’ cognitive and temperamental development. J. Affect. Disord. 2017, 207, 9-17. doi:10.1016/j.jad.2016.09.010.
- Smith, G. C.; Gutovich, J.; Smyser, C.; Pineda, R.; Newnham, C.; Tjoeng, T. H.; Inder, T. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann. Neurol. 2011, 70 (4), 541-549. doi:10.1002/ana.22545.
- Smyser, C. D.; Inder, T. E.; Shimony, J. S.; Hill, J. E.; Degnan, A. J.; Snyder, A. Z.; Neil, J. J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex 2010, 20 (12), 2852-2862.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph20032718
