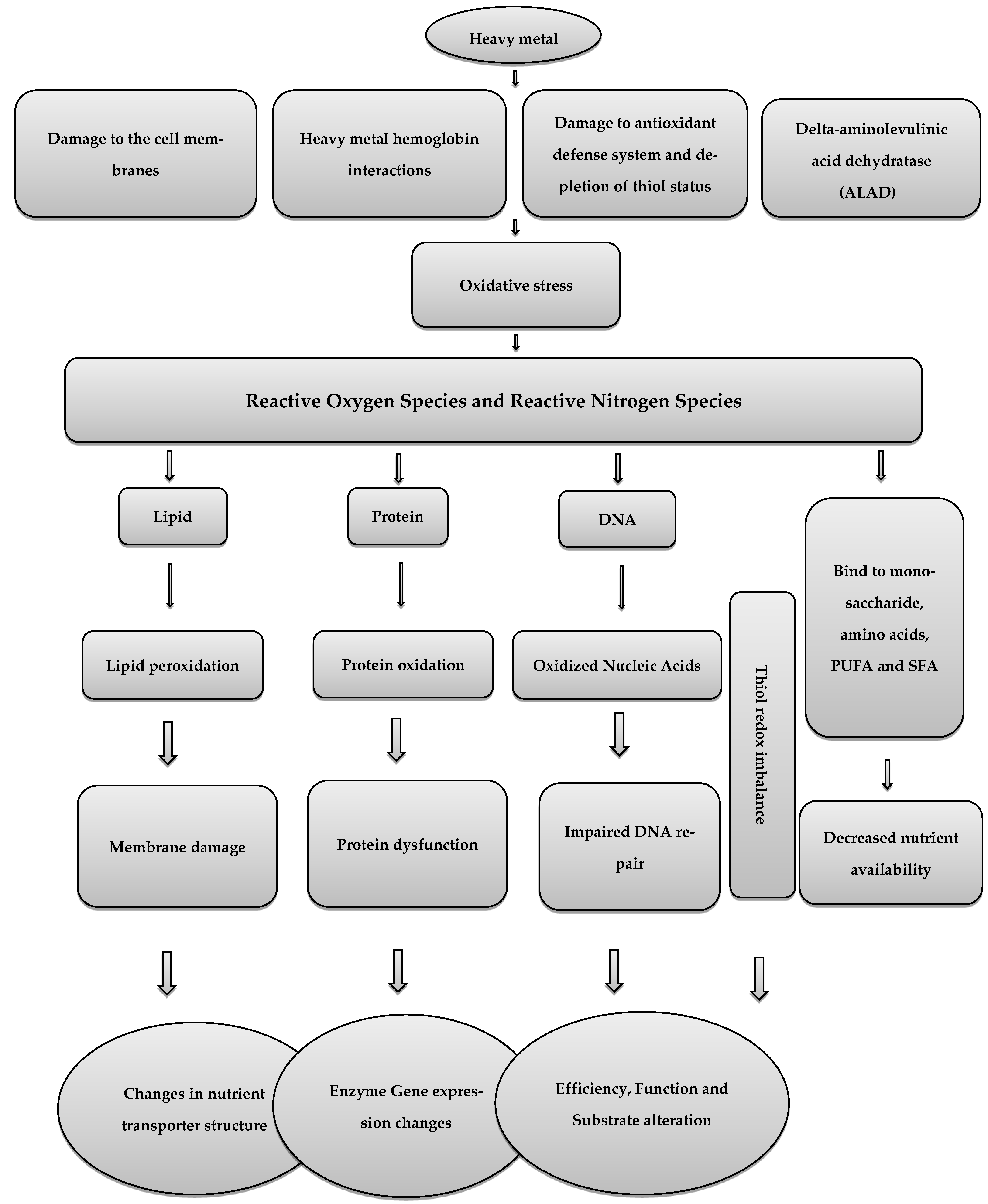
2. Heavy Metals and Oxidative Stress in Poultry
2.1. Pb
Because of Pb’s non-biodegradable nature and the ongoing use of items related to it, there are severe challenges to global food security [
20]. Poultry is one of the most important agricultural sectors, and each year, more than 50 billion birds are raised for food [
21]. On broiler growth, even 1.0 mg/kg of trace Pb concentration in feed can have a significant effect, including linear decreases in body weight gain, linear decrease in delta-aminolevulinic acid dehydratase (ALAD) activity and dose-related increase in Pb in the blood, kidney, liver and tibia in broiler chickens [
22]. Two healthy elements of eggs, ovalbumin and phosvitin, have been proven to bind a variety of metals [
23], increasing the risk of heavy metal accumulation in eggs. The rate of Pb transmission from female birds to eggs was assumed to be highly associated with the quantities in livers [
24]. A previous investigation suggested that Pb may alter the way Ca is digested, making it easier for Pb to be incorporated into eggshells [
25]. Therefore, egg production may offer a means for female birds to eliminate Pb other than through urine or feces [
26]. A study showed that Pb can lead to oxidative stress in chickens and hasten biological component oxidation processes by generating free radicals [
27]. The key contributor to the ionic mechanism of Pb poisoning, which eventually interferes with cell metabolism, is the ability of Pb metal ions to replace other bivalent cations such as Ca
2+, Mg
2+, and Fe
2+ as well as monovalent cations such as Na
+. The ionic mechanism of Pb poisoning significantly affects a wide range of biological activities, including cell adhesion, intracellular and intercellular communication, protein folding, maturation, apoptosis, ionic transport, enzyme control, and neurotransmitter release. Protein kinase C, a gene that regulates neuronal excitation and memory storage, is impacted by the fact that Pb can replace calcium even in picomolar quantities [
28]. Pb ions may alter the morphology and operation of the mitochondria, which would cause cell death. The induction of the mitochondrial permeability transition pore (mPTP) is significantly influenced by the production of ROS [
29]. The accumulation of ROS brought on by Pb, however, has been shown to impact energy metabolism and alter DNA through point mutations, rearrangements, and fragmentation [
29]. It has also proposed that the disruptive effects of Pb ions on mitochondrial respiratory complexes are the root cause of Pb (II)-induced liver damage. The release of cytochrome C and opening the permeability transition pore (PTP), which results in mitochondrial dysfunction and possibly cell death signaling, is what causes the toxicity [
30]. Overall, Pb can cause oxidative damage in various organs by having a direct impact on the peroxidation of membrane lipids and lowering antioxidant properties [
31]. The antioxidant parameters GPx, CAT, SOD, glutathione S—Transferase (GST), and glutathione (GSH) are considerably decreased by exposure to Pb, whereas the oxidative parameters MDA and H
2O
2 are increased [
32]. In Pb poisoning, caspase-3 is activated once mitochondrial cytochrome C is released, which inhibits Bcl-2 and causes apoptosis [
33]. Additionally, blocking the release of neurotransmitters and inhibiting the N-methyl-D-aspartate (NMDA) receptor may cause Pb neurotoxicity and cognitive impairment [
34].
2.2. Cd and As
Hazardous heavy metal Cd is widely present in nature and has a variety of adverse effects for human/animal health. The creation of nickel-cadmium batteries, electroplating, burning of fossil fuels, and mining waste are all examples of human activities that contribute to the widespread occurrence of Cd [
9]. Due of these factors, almost everything we ingest, such as food, drinks, and air, includes Cd [
10]. Continuous exposure to low levels of Cd causes daily buildup in several tissues and adverse effects on various organs [
8]. Due to its chemical similarity, it can mimic and substitute some nutritional metals in a range of biological structures [
35]. Recent research suggests that Cd may be dangerous to health even at low dosages because of its propensity to accumulate [
36]. For animal husbandry in some countries, Cd contamination has been a problem, and in some instances, the amount of Cd in manure and animal feed can even approach 130 mg/kg [
37]. Long-term exposure to Cd can result in hepatotoxicity, renal failure, and neurotoxicity [
38]. The rapid impacts of Cd exposure in vivo include the generation of ROS in the mitochondrial electron transfer chain being stimulated, NADPH oxidase activity in the plasma being inhibited, and physiological antioxidants such as glutathione being depleted. The buildup of Cd in tissues could slow the pace of growth [
39]. The kidney and liver are the main tissue targets for Cd toxicity. Cd produces oxidative stress by increasing the formation of free radicals. Increased ROS can lead to lipid peroxidation, DNA oxidation, sulfhydryl depletion, and a disruption of calcium homoeostasis [
40]. The specific mechanism of Cd poisoning is unknown, although its effects on cells are widely known [
41]. Cd concentration increases by a factor of 3000 when it binds to metallothionein or other proteins with a high cysteine content. After causing hepatotoxicity in the liver, the cysteine–metallothionein complex builds up in the renal tissue of the kidney and causes nephrotoxicity. Aspartate, glutamate, histidine, and cysteine are also among the ligands that Cd can bind, and this can cause an iron deficiency [
42]. Due to their similar oxidation states, Cd and zinc could have been substituted in metallothionein to prevent it from acting as a cell’s free radical scavenger. Cd and Pb both have the capacity to mimic significant metals and/or replace them if necessary. These ions attach to calmodulin [
43], protein kinase C [
44], troponin C, and synaptic proteins [
45] that include magnesium-, zinc-, and calcium-specific binding sites. The most stable complexes are created when these so-called “soft metals” join forces with the mixed N-S donor atom ligand. Recent research on mouse renal tubular epithelial cells has shown that Cd can trigger the release of Ca (II) from ER reserves through the phospholipase C (PLC)-IP3 pathway, which participates in the formation of ROS [
46]. Cd can bind to lipids, proteins, and nucleic acids after entering the body. Thiol (-SH) groups are frequently used for binding to enzymes and proteins, and they modify cysteine residues in proteins. This type of protein inactivation has the power to disturb the intracellular redox balance. As a result, liver harm develops as a result of an unbalanced antioxidant defense [
7]. Additionally, it has been thought that Cd indirectly produces ROS. The antioxidant defense of cells could be overwhelmed by O
2, hydroxyl (OH), and the NO radicals that Cd could indirectly produce [
47]. This might be the result of cellular proteins containing more Cd than iron and copper. The buildup of too much Fe and Cu is the cause of the oxidative stress. Additionally, replacing the necessary minerals throws off the cellular metabolism of the cell. Alternately, Cd might interfere with glutathione, causing oxidative stress to develop [
48].
Long-term exposure to arsenic has been demonstrated to be harmful to the liver, lung epithelial transformed cells, and skin, and it has also been shown that arsenic alters multiple cellular pathways, including cytokine expression, apoptosis promotion and resistance, and increased oxidative stress [
49]. These alterations result in the manifestation of disease [
50]. In chicken hearts, subchronic arsenism-induced oxidative stress is also thought to trigger inflammation, and it is believed that ROS overproduction activates the NF-B pathway, which in turn causes an increase in the expression of pro-inflammatory mediators such as TNF, prostaglandin E synthase, cyclooxygenase-2, and inducible nitric oxide synthase [
50]. Arsenic pathogenesis is characterized by oxidative damage caused by ROS. Arsenic also causes morphological abnormalities in the mitochondria’s structural integrity. Cells are more susceptible to the harmful effects of arsenic as a result of glutathione-depleting substances paired with cascade mechanisms of free radical generation resulting from the superoxide radical. The formation of ROS/RNS, including peroxyl radicals, the superoxide radical, singlet oxygen, hydroxyl radicals via the Fenton reaction, hydrogen peroxide, the dimethylarsenic radical, the dimethylarsenic peroxyl radical, and/or oxidant-induced DNA damage, is increased in both humans and animals exposed to As [
51].
2.3. Mitigation of Oxidative Stress in Poultry
Dietary antioxidants help to keep the intestinal mucosa healthy while lowering intestinal free radicals [
17]. Numerous studies indicate that oxidative stress rids birds of various pathogenic and welfare problems [
52]. Therefore, the poultry industry must design a practical strategy to prevent oxidative stress [
4]. To reduce oxidative distress in poultry [
53], a variety of dietary therapies are available based on the best supplementation of antioxidative vitamins (E, A, C, and B
2) and micronutrients (Se, Cu, and Zn). Researchers have recently become interested in the chemicals discussed below because of their chelating and growth-stimulating functions, as well as the antioxidant qualities of useful plant components.
The liver is known to be protected by polyphenolic compounds from a variety of xenobiotics, including Pb and diethyl nitrosamine, which can cause hepatotoxicity [
54]. Over the past ten years, many in vitro and in vivo studies have suggested that tea and tea polyphenols have potent antioxidant activity as well as a variety of other potentially useful medicinal properties, including the capacity to inhibit tumor growth, metastasis and carcinogenesis in various animals. The main polyphenolic components in tea are catechins. Tea catechins are effective scavengers of superoxide, hydrogen peroxide, hydroxyl radicals, and nitric oxide in various compounds. They were also able to bind with metals, thanks to their catechol structure [
55]. The protective effect tea catechins exert on oxidative damage in HepG2 cells exposed to Pb may be due to their capacity to bind metal ions and scavenge free radicals [
56]. According to [
57], experiments are being conducted to find ways to lessen the harmful effects of Cd and Pb on the body by chelating these metals with nutrients, which reduces their absorption by tissues or boosts the body’s oxidative capacity. However, there are presently no effective techniques to reduce the levels of Cd and Pb in food and hence lessen the risk of oxidative stress being induced in internal organs. Supplemental lycopene and fucoxanthin shield rat kidney, bone, and brain tissue against the effects of Cd-mediated oxidative stress [
58].
Recent studies have used astaxanthin, a red carotenoid pigment found in some marine species and a potent antioxidant without provitamin-A activity, to improve rooster sperm quality [
59]. According to [
60], at a concentration of 25 mg/kg, astaxanthin nanoparticles act as a potent antioxidant to shield rooster testes from the oxidative stress caused by Cd injection and maintain the post-thawing quality of rooster sperm. As with other carotenoids, astaxanthin has a low bioavailability despite being a highly lipophilic molecule. As a result, an astaxanthin nano preparation that is more stable and bioavailable has been developed. However, the study investigates how astaxanthin, a lipid-soluble carotenoid, protects against Cd-induced damage to rooster testis and decreased sperm quality. Additionally, it has been noted in the literature that astaxanthin has around 100 times the antioxidant activity of alpha-tocopherol and about 10 times the antioxidant activity of other carotenoids, including zeaxanthin, lutein, canthaxanthin, and beta-carotene [
61].
It has been demonstrated that the polyphenolic molecule resveratrol has strong antioxidant properties that can protect against hydroxyl and superoxide radicals produced by heavy metals [
62]. Additionally, it may increase the activity of GSH-Px, CAT, GST, SOD, and nicotinamide adenine while activating the key transcription factors that regulate the response to antioxidants (erythroid-derived nuclear factor) [
63]. It might also maintain glutathione in its reduced state by blocking the creation of glutathione disulfide. This would prevent the oxidation of macromolecules, inhibit the peroxidation of the apolipoprotein B protein, and protect cells from the onslaught of free radicals [
64]. Resveratrol has also been demonstrated to lessen oxidative stress and increase antioxidant status in chickens when provided as a dietary supplement [
65]. Additionally, yucca’s resveratrol and other phenolic components may reduce lipid peroxidation and stop the generation of blood platelet free radicals (LPO) [
66]. It might also maintain glutathione in its reduced state by stopping the manufacture of glutathione disulfide [
67]. Chelation therapy, antioxidant therapy, and the consumption of natural food components are among the therapeutic and preventive approaches available to combat As toxicity. Natural dietary substances and medications with a plant-based origin provide effective and progressive treatment from As-mediated toxicity without causing any distinct side effects. Due in significant part to their robust antioxidant properties, bioactive compounds have generated considerable interest in their potential advantages [
68]. In a recent study, 34 medicinal plants and 14 natural compounds, largely in preclinical trials and a few in clinical research, demonstrated considerable protection against As toxicity [
69]. According to [
70],
Allium sativum,
Curcuma longa,
Silybum marianum, as well as various herbal fibers and algae, are the most effective medicinal plants for treating As toxicity. Organosulfur-containing vegetables are helpful in removing arsenic from the liver. Dialyl sulphide, an organosulfur natural substance present in garlic (
Allium sativum), has been shown to reduce toxicity and As-induced mitochondrial dysfunction in mice [
71]. Crude extracts of Viscum album and
Allium sativum were investigated for their ability to counteract in vivo experimentally generated As toxicity [
72]. Experimental evidence of the antioxidant properties of members of the Lamiaceae family was found in Ocimum sanctum leaf extract [
73]. Due to its antioxidant activity, Silybum marianum’s flavonolignan, silibinin, exerts beneficial effects on rats exposed to As [
74].