Increases in temperature and the greater incidence of extreme events are the consequences of the climate change that is taking place on planet Earth. High temperatures create severe discomfort to animal farms as they are unable to efficiently dissipate their body heat, and for this, they implement mechanisms to reduce the production of endogenous heat (reducing feed intake and production). In tropical and subtropical countries, where buffalo breeding is more widespread, there are strong negative consequences of heat stress (HS) on the production and quality of milk, reproduction, and health. The increase in ambient temperature is also affecting temperate countries in which buffalo farms are starting to highlight problems due to HS.
- dairy buffalo
- heat stress
- feeding
- milk yield and composition
1. Heat Stress Introduction
2. Mitigation Strategies
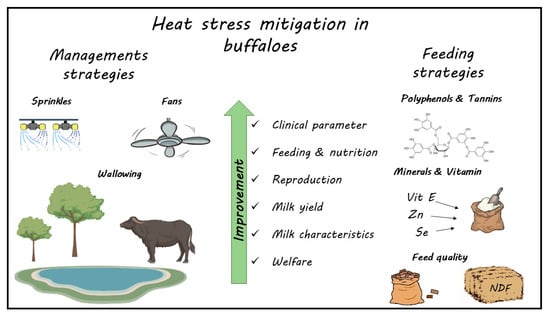
2.1. Feeding and Nutritional Strategies
| References | Breed | Treatment | RT | ST | RR | HR | DMI | MY | MQ |
|---|---|---|---|---|---|---|---|---|---|
| [29] | Med. Italian | Zn + Se | n.e. | ↑* | n.e. | ||||
| [30] | Surti | Vit B3 | ↓* | ↓* | ↓* | n.e. | ↑* | ↑* | |
| [32] | Egyptian | Moringa oleifera leaves | ↓* | ↓* | ↓* | ↓* | |||
| [33] | Murrah | 34.5% NDF and 7% MP | n.e. | ↓* | n.e. | n.e. | n.e. | ↑* | |
| 37% NDF and 7% MP | n.e. | n.e. | ↓* | n.e. | n.e. | ↑* | |||
| 30% NDF and 8% MP | ↓* | ↓* | ↓* | n.e. | n.e. | ↑* | |||
| 34.5% NDF and 8% MP | ↓* | ↓* | ↓* | n.e. | n.e. | ↑* | |||
| 37% NDF and 8% MP | ↓* | ↓* | ↓* | n.e. | n.e. | ↑* | |||
| [34] | Murrah | +15% ME | n.e. | n.e. | n.e. | ||||
| −15% ME | ↑* | ↑* | n.e. |
2.2. Cooling Strategies
| References | Breed | Treatment Type | FB | CP | SI | MY | MC | RC | DMI | WI |
|---|---|---|---|---|---|---|---|---|---|---|
| [36] | Mediterranean Italian | Free stall open-sided barn with an outdoor lot and a concrete pool | n.e. | ↑** | ↑* | n.e. | n.e. | |||
| [39] | Nili-Ravi | Shade with a fan | ↑* | ↑* | ↑* | ↓* | ||||
| Shade, a fan, and sprinklers | ↑* | ↑* | ↑* | ↓* | ||||||
| [14] | Murrah | Misting | ↓* | ↑* | ||||||
| Wallowing | ↓* | n.e. | ||||||||
| [42] | Murrah | Silvopastoral system | ↑* | ↓* | ||||||
| [38] | Murrah | Wallowing | ↓* | ↑* | ↑* | |||||
| [40] | Nili-Ravi | Shade with a fan | ↓* | n.e. | ↑* | ↑* | ↓* | |||
| Shade, a fan, and sprinklers | ↓* | ↑* | ↑* | ↑* | ↓* |
This entry is adapted from the peer-reviewed paper 10.3390/ani13071260
References
- IPCC. Working Group III contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; p. 1435.
- IPCC. Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2022: Impacts, Adaptation and Vulnerability; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022.
- Zobeidi, T.; Yaghoubi, J.; Yazdanpanah, M. Farmers’ Incremental Adaptation to Water Scarcity: An Application of the Model of Private Proactive Adaptation to Climate Change (MPPACC). Agric. Water Manag. 2022, 264, 107528.
- Segnalini, M.; Bernabucci, U.; Vitali, A.; Nardone, A.; Lacetera, N. Temperature Humidity Index Scenarios in the Mediterranean Basin. Int. J. Biometeorol. 2013, 57, 451–458.
- Segnalini, M.; Nardone, A.; Bernabucci, U.; Vitali, A.; Ronchi, B.; Lacetera, N. Dynamics of the Temperature-Humidity Index in the Mediterranean Basin. Int. J. Biometeorol. 2011, 55, 253–263.
- Bernabucci, U.; Biffani, S.; Buggiotti, L.; Vitali, A.; Lacetera, N.; Nardone, A. The Effects of Heat Stress in Italian Holstein Dairy Cattle. J. Dairy Sci. 2014, 97, 471–486.
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat Stress: Physiology of Acclimation and Adaptation. Anim. Front. 2019, 9, 12–19.
- Bernabucci, U.; Lacetera, N.; Baumgard, L.H.; Rhoads, R.P.; Ronchi, B.; Nardone, A. Metabolic and Hormonal Acclimation to Heat Stress in Domesticated Ruminants. Animal 2010, 4, 1167–1183.
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s Biological Functions as Affected by Heat Stress—A Review. Livest. Sci. 2010, 127, 89–109.
- Matera, R.; Cotticelli, A.; Gómez Carpio, M.; Biffani, S.; Iannacone, F.; Salzano, A.; Neglia, G. Relationship among Production Traits, Somatic Cell Score and Temperature–Humidity Index in the Italian Mediterranean Buffalo. Ital. J. Anim. Sci. 2022, 21, 551–561.
- Debbarma, D.; Uppal, V.; Bansal, N.; Gupta, A. Histomorphometrical Study on Regional Variation in Distribution of Sweat Glands in Buffalo Skin. Dermatol. Res. Pract. 2018, 2018, 5345390.
- Shafie, M. Physiological Responses and Adptation of Water Buffalo. In Stress Physiology in Lifestocks; CRC: Boca Raton, FL, USA, 1985; Volume 2, Ungulates.
- Shafie, M.; El-Khair, A. Activity of the Sebaceous Glands of Bovines in Hot Climate. Egypt. J. Anim. Prod. 1970, 10, 81–98.
- Yadav, B.; Pandey, V.; Yadav, S.; Singh, Y.; Kumar, V.; Sirohi, R. Effect of Misting and Wallowing Cooling Systems on Milk Yield, Blood and Physiological Variables during Heat Stress in Lactating Murrah Buffalo. J. Anim. Sci. Technol. 2016, 58, 2.
- Aggarwal, A.; Singh, M. Changes in Skin and Rectal Temperature in Lactating Buffaloes Provided with Showers and Wallowing during Hot-Dry Season. Trop. Anim. Health Prod. 2008, 40, 223–228.
- Santhosh Kumar, V.; Sahithya Rani Asssitant professor, M.; Prasanna Kumar, R.; Harikrishna, C.; Sahithya Rani, M. Effect of Heat Stress on Production and Reproduction Performance of Buffaloes-A Review. Pharma Innov. J. 2018, 7, 629–633.
- Costa, A.; Neglia, G.; Campanile, G.; de Marchi, M. Milk Somatic Cell Count and Its Relationship with Milk Yield and Quality Traits in Italian Water Buffaloes. J. Dairy Sci. 2020, 103, 5485–5494.
- Upadhyay, R.C.; Singh, S.; Kumar, A.; Gupta, S.K.; Ashutosh, A. Impact of Climate Change on Milk Production of Murrah Buffaloes. Ital. J. Anim. Sci. 2007, 6, 1329–1332.
- Mullick, D.N. Effect Oh Humidity and Exposure to Sun on the Pulse Rate, Respiration Rate, Rectal Temperature and Haemoglobin Level in Different Sexes of Cattle and Buffalo. J. Agric. Sci. 1960, 54, 391–394.
- Ji, B.; Banhazi, T.; Perano, K.; Ghahramani, A.; Bowtell, L.; Wang, C.; Li, B. A Review of Measuring, Assessing and Mitigating Heat Stress in Dairy Cattle. Biosyst. Eng. 2020, 199, 4–26.
- Choudhary, B.B.; Sirohi, S. Sensitivity of Buffaloes (Bubalus Bubalis) to Heat Stress. J. Dairy Res. 2019, 86, 399–405.
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of Heat Stress on Reproductive Performances of Dairy Cattle and Buffaloes: A Review. Vet. World 2016, 9, 235–244.
- Mishra, S.R. Thermoregulatory Responses in Riverine Buffaloes against Heat Stress: An Updated Review. J. Therm. Biol. 2021, 96, 102844.
- Gudev, D.; Popova-Ralcheva, S.; Moneva, P.; Aleksiev, Y.; Peeva, T.; Penchev, P.; Ilieva, I. Physiological Indices in Buffaloes Exposed to Sun. Arch. Zootech. 2007, 10, 127–133.
- Mostageer, A.; Obeidah, A.M.; Shafie, M.M. A Statistical Study of Some Physiological Factors Affecting Body Temperature and Respiration Rate in Buffaloes and Friesian Cattle. Z. Für Tierzüchtung Züchtungsbiologie 1974, 91, 327–333.
- Shenhe, L.; Jun, L.; Zipeng, L.; Tingxian, D.; Rehman, Z.U.; Zichao, Z.; Liguo, Y. Effect of Season and Breed on Physiological and Blood Parameters in Buffaloes. J. Dairy Res. 2018, 85, 181–184.
- Nasr, M.A.F. The Impact of Crossbreeding Egyptian and Italian Buffalo on Milk Yield and Composition under Subtropical Environmental Conditions. J. Dairy Res. 2016, 83, 196–201.
- Megahed, G.A.; Anwar, M.M.; Wasfy, S.I.; Hammadeh, M.E. Influence of Heat Stress on the Cortisol and Oxidant-Antioxidants Balance during Oestrous Phase in Buffalo-Cows (Bubalus Bubalis): Thermo-Protective Role of Antioxidant Treatment. Reprod. Domest. Anim. 2008, 43, 672–677.
- Evangelista, C.; Bernabucci, U.; Basiricò, L. Effect of Antioxidant Supplementation on Milk Yield and Quality in Italian Mediterranean Lactating Buffaloes. Animals 2022, 12, 1903.
- Chaudhary, S.S.; Singh, V.K.; Manat, T.D.; Patel, S.B.; Patel, N.B. Heat Ameliorative Effects of Rumen Protected Niacin Supplementation in Lactating Surti Buffaloes. Buffalo Bull. 2022, 41, 773–785.
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Markers of Oxidative Status in Plasma and Erythrocytes of Transition Dairy Cows during Hot Season. J. Dairy Sci. 2002, 85, 2173–2179.
- Wafa, W.M.; El-Nagar, H.A.; Gabr, A.A.; Rezk, M.M. Impact of Dietary Moringa Oleifera Leaves Supplementation on Semen Characteristics, Oxidative Stress, Physiological Response and Blood Parameters of Heat Stressed Buffalo Bulls. J. Anim. Poult. Prod. 2017, 8, 367–379.
- Lakhani, N.; Tyagi, N.; Agarwal, A.; Kumar, S.; Tyagi, A. Optimizing Fiber and Protein Levels in Diet of Lactating Murrah Buffaloes to Ameliorate Heat Stress: Effect on Physiological Status and Production Performance. J. Therm. Biol. 2021, 96, 102838.
- Talukdar, P.; Kundu, S.; Mondal, G. Influence of Temperature Humidity Index and Dietary Energy on Certain Stress Marker Profile of Murrah Buffalo Heifers. Int. J. Livest. Res. 2017, 1, 195–204.
- Kumar, S.; Singh, S.K.; Srivastava, A.K. Effect of I/M Se and Vit E Administration Combined with Cooler Hours Feeding Regime on Milk Production in Buffaloes in Hot–Humid Climate of Bundelkhand Region. Indian J. Anim. Res. 2019, 53, 1258–1260.
- de Rosa, G.; Grasso, F.; Braghieri, A.; Bilancione, A.; di Francia, A.; Napolitano, F. Behavior and Milk Production of Buffalo Cows as Affected by Housing System. J. Dairy Sci. 2009, 92, 907–912.
- Neglia, G.; Rendina, M.; Balestrieri, A.; lo Grasso, F.; Potena, A.; Russo, I.; Zicarelli, L. Influence of a Swimming-Pool on Fertility in Buffalo Species. Ital. J. Anim. Sci. 2009, 8, 637–639.
- Aggarwal, A.; Singh, M. Hormonal Changes in Heat-Stressed Murrah Buffaloes under Two Different Cooling System. Buffalo Bull. 2010, 29, 1–6.
- Ahmad, M.; Bhatti, J.A.; Abdullah, M.; Javed, K.; Din, U.; Ali, M.; Rashid, G.; Ahmed, N.; Jehan, M. Effect of Different Ambient Management on Milk Production and physiological Performance of Lactating Nili- Ravi Buffaloes during Hot Humid Summer. Livest. Res. Rural. Dev. 2017, 29, 230.
- Ahmad, M.; Bhatti, J.A.; Abdullah, M.; Ullah, R.; ul Ain, Q.; Hasni, M.S.; Mahboob, A.; Rashid, A.; Qaisar, I.; Rashid, G.; et al. Different Ambient Management Intervention Techniques and Their Effect on Milk Production and Physiological Parameters of Lactating NiliRavi Buffaloes during Hot Dry Summer of Subtropical Region. Trop. Anim. Health Prod. 2019, 51, 911–918.
- Hoque, M.R.; Rana, M.S.; Nayan, S.B.; Miraz, M.F.H.; Deb, G.K.; Nahar, T.N.; Habib, R.; Siddiki, M.S.R. Influence of Multiple Showering on Quality of Buffalo Semen during Hot-Humid Season. J. Adv. Vet. Anim. Res. 2018, 5, 12–18.
- Athaíde, L.G.; Joset, W.C.L.; de Almeida, J.C.F.; Pantoja, M.H.d.A.; Noronha, R.d.P.P.; Bezerra, A.S.; Barbosa, A.V.C.; Martorano, L.G.; da Silva, J.A.R.; Lourenço Júnior, J.d.B. Thermoregulatory and Behavioral Responses of Buffaloes With and Without Direct Sun Exposure during Abnormal Environmental Condition in Marajó Island, Pará, Brazil. Front. Vet. Sci. 2020, 7, 522551.
- Ramadan, T.A.; Taha, T.A.; Samak, M.A.; Hassan, A. Seasonal and Monthly Variations in Semen Characteristics of Egyptian Buffalo Bulls. Lexandria J. Agric. Sci. 2009, 54, 13–23.
- Gonçalves, A.A.; Garcia, A.R.; Rolim Filho, S.T.; da Silva, J.A.R.; de Melo, D.N.; Guimarães, T.C.; Tavares, H.R.; Silva, T.V.G.; de Souza, E.B.; Santos, S.d.S.D.; et al. Scrotal Thermoregulation and Sequential Sperm Abnormalities in Buffalo Bulls (Bubalus Bubalis) under Short-Term Heat Stress. J. Therm. Biol. 2021, 96, 102842.
- Sharma, M.; Bhat, Y.; Sharma, N.; Singh, A. Comparative Study of Seasonal Variation in Semen Characteristics of Buffalo Bull. J. Entomol. Zool. Stud. 2018, 6, 947–951.
- Singh, A.K.; Devi, R.; Kumar, Y.; Kumar, P.; Upadhyay, R.C. Physiological Changes and Blood Flow in Murrah Buffaloes during Summer and Winter Season. J. Buffalo Sci. 2014, 3, 63–69.
- Galloso-Hernández, M.A.; Soca-Pérez, M.; Dublin, D.; Alvarez-Díaz, C.A.; Iglesias-Gómez, J.; Díaz-Gaona, C.; Rodríguez-Estévez, V. Thermoregulatory and Feeding Behavior under Different Management and Heat Stress Conditions in Heifer Water Buffalo (Bubalus Bubalis) in the Tropics. Animals 2021, 11, 1162.
