Hydrolysis generally refers to the breakdown of polymeric substance into their monomeric building blocks. In the case of microbial hydrolysis, the breakdown is catalyzed by extracellular enzymes produced by hydrolytic microorganisms. This article focusses on microbial hydrolysis within the process of anerobic digestion including the relevant metabolites, microbial consortia and the role of hydrolysis in anerobic digestion systems.
- biomass pretreatment
- hydrolysis stage
- multi-stage digestion
- biological pretreatment
- biodegradable waste
- Biorefinery
- Anaerobic digestion
1. Introduction to multi-stage anaerobic digestion
Anaerobic digestion can produce bioenergy and value-added products from biodegradable residues; it represents an approach to close the loop in a circular bioeconomy. Examples are summarized in [1],[2] among others. Anaerobic digestion is a well-established and mature technology in Europe with more than 18,000 production plants in 2018 that are already providing about 14% of renewable energy. Moreover, as calculated by the World Biogas Association, the biogas and biomethane sector of anaerobic digestion can potentially reduce greenhouse gas emissions by 10–13%[3].
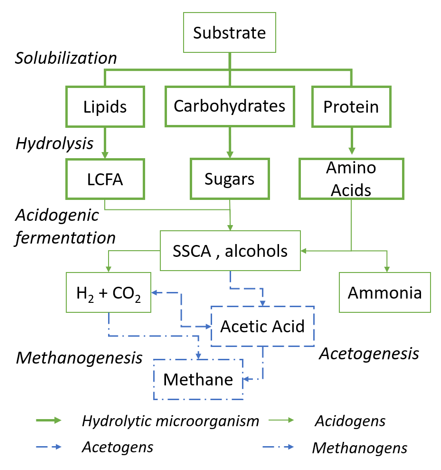
In brief, anaerobic digestion of biodegradable residues consists of four metabolic stages, as shown in Figure 1. During hydrolysis, bulk biomass is degraded to soluble carbohydrates, proteins and lipids followed by acidogenesis, where these are converted mainly to short-chain carboxylic acids (SCCA) and alcohols. In acetogenesis and methanogenesis, acetic acid is consumed or assimilated and converted to methane and carbon dioxide. For the vast majority of biodegradable waste, hydrolysis represents the process bottleneck due to slow rates and incomplete degradation [4][5]. Many reviews have been published on pretreatment methods of various biogenic residues to improve hydrolysis which include mechanical, thermal, chemical, biological and mixed pretreatments. The most promising of these methods concerning hydrolysis efficiency need high-energy inputs (e.g., thermal/microwave treatment) or huge amounts of chemicals (acidic pretreatment), which makes the process costly in industrial scale [4][5]. The introduction of a separate reactor stage focused on microbial hydrolysis in anaerobic digestion has already shown to increase the net energy output in pilot scale fermentations, making it a promising as potential biological pretreatment. Separation of the hydrolysis–acidogenesis and acetogenesis–methanogenesis enables the optimization of process parameters to the different conditions of the corresponding microorganism, enabling higher efficiency in hydrolysis and methanogenesis[6][7][8][9]. While hydrolytic and acidogenic microorganisms favor a slightly acidic pH around 5.0–6.0, the methanogenic species are rather sensitive and thrive at neutral pH and mesophilic conditions. There are some examples of a separate hydrolysis and acidogenesis in a three stage AD, the strong syntrophic relationship between acetogens and methanogens makes the separation of the last stages adverse [1][2].
Since currently more complex and unsteady sources of organic residues are used as substrates for AD, flexibilization of AD is of high importance. Microbial communities in a hydrolysis stage are able to adapt to changing substrates and loading rates and are able to digest the biogenic residues under optimal process conditions [10].
Figure 1. Metabolic pathways of anaerobic digestion and involved microorganisms. LCFA—long-chain fatty acids, SCCA—short chain carboxylic acid
Microbial Consortia and the Main Products in a Microbial Hydrolysis Stage
The substrates for anaerobic digestion are very diverse and can roughly be divided into three groups: liquid residues, solid residues and lignocellulosic residues. While all the substrates are biodegradable, they feature very different biochemical properties and composition. Substrates high in easily digestible sugars as food waste are easier to digest than lignocellulosic substrates like grass silage. Nonetheless, all contain polymers that need to be hydrolyzed[11].
These polymeric substances (lipids, proteins, carbohydrates) in the substrate are broken down into low molecular-weight intermediates with molecular weight < 1000 Da by extracellular enzymes, which are secreted by the microbial community. The intermediates are then taken up by the cells. The extracellular hydrolases as e.g., lipases, proteases and glucosidases are expressed free into the fluid phase, bound to microbial membranes or immobilized in a multi-enzyme complex called the cellulosome [12]. Cellulolytic microorganisms have developed complex enzyme systems for the hydrolysis of recalcitrant cellulosic biomass, as discussed by Himmel and co-workers [13]. Enzyme expression and enzymatic activity are of high significance for the hydrolytic step in anaerobic digestion [12]. Some intermediates produced in hydrolysis as ammonia and LCFA were found to have inhibiting effects on the anerobic digestion process and require acclimatization of the microbial community or operational strategies for efficient digestion. Moreover, hydrolytic organisms metabolize the monomers produced by hydrolytic enzymes and concurrently produce intermediates from acidogenesis as SCCA and hydrogen.
Anaerobic digesters are usually inoculated with a mixed microbial consortia from running fermentations. Depending on parameters like substrate, inoculum and environmental growth conditions, the microbial community develops over time. The hydrolysis stage is usually dominated by bacteria like Bacteroidetes, Firmicutes and Proteobacteria. However, changing process conditions and substrates promote the enrichment of various microorganisms [14][1]. Among them, anaerobic fungi like Neocalimastix, Piromyces and Orpinomyces play an important role in hydrolysis and fiber degradation due to a strong multi-enzyme system to degrade cellulosic material [1][15]. Moreover, aerobic fungi like white-rot fungi are common for digestion of lignocellulosic biomass [15]. In the hydrolysis stage, different metabolic types of fermentation have been identified with their bacterial key players. Butyric type fermentation with high production of acetic acid, butyric acid and hydrogen with dominance of Clostridium is considered best for two-stage AD and can be influenced by process parameters as pH, organic loading rate (OLR), oxidation–reduction potential [16] and hydraulic retention time (HRT) [17][18]. The influence of OLR seems to vary with the substrate, but various groups found that pH > 5.0 favors butyric type fermentation, whereas a lower pH leads to dominance of Lactobacilli producing lactic acid or ethanol metabolism [14][16][19]. Ethanol-type fermentation is observed mostly at pH 4.0–4.5 and may feature high gas production [14][20]. More alkaline pH favors production of acetic acid while inhibiting growth of methanogens [21]. Propionic acid is another mayor fermentation product. Depending on the substrate, it can reach a proportion of 20–40%, predominantly in AD of protein-rich waste as sunflower oil cake [22] or waste activated sludge [23]. It has been found that in the AD of carbohydrate-rich substrate, acetate and propionate are dominant at mesophilic temperature and acidic pH, whereas the fermentation shifts to butyrate at thermophilic AD [21][24]. While the fermentation type is always substrate-dependent, the pH plays a significant role in selecting the fermentation type and the distribution of SCCA [21][25][14]. Since butyric type fermentation is considered best for two-stage AD, pH regulation towards pH 5.0–6.0 in the hydrolysis stage plays a significant role for total process performance.
Stage separation of AD allows the enrichment of specific microorganism in each digester. The second stage of AD is dominated by methanogenic archaea that have a higher sensitivity for process conditions like temperature and pH than the hydrolytic bacteria. pH below 6.5 and thermophilic temperature decrease archaea diversity [1][26]. In a study of Hameed et al., the abundance of Methanosarcina—assumed to be a main producer of methane in biogas production—was reduced from 76.7% to 23.8%, and also the alpha diversity decreased with a shift from 35 °C to 55 °C [27]. As a diverse microbiome is assumed to be more stable, staging of AD leads to a better process performance. Hydrolysis rate and deactivation of pathogens are increased with a rising temperature, making a thermophilic process (50 °C–60 °C) attractive. The traditional mesophilic process (30 °C–40 °C) offers higher OLR, better stability and less energy requirements. A good compromise seems to be the temperature-phased AD with a thermophilic hydrolysis stage and mesophilic second stage [1].
The knowledge about microbial communities and metabolic pathways in AD is very important to understand and adapt the process conditions for the desired products. The authors would like to refer to the review on the dynamics of the microbiome in AD by the group of Castellano-Hinojosa et al. for further information [1].
3. Microbial Hydrolysis in a Separate Stage
Reviews on multi-stage AD systems for biodegradable waste have been published by Van et al. [2] and Chatterjee and Mazumder [28] who conclude, that two-stage AD is a robust, flexible and efficient system offering shorter hydraulic retention times (HRT), higher organic loading rates (OLR), higher digester efficiency and higher gas yields compared to single-stage AD. Two-stage AD is most efficient for biogenic residues with a solid content between 3–20%. Below a solid content of 3%, the single-stage AD is more efficient in terms of energy efficiency, whereas for high solid substrate, there is a significant increase in required energy input to dilute the substrate in a continuously stirred tank reactor (CSTR) system [2][28]. The process is more resistant to shock loadings and variable substrate, making it a promising system for the application in biorefinery systems [28]. The better hydrolysis and acidogenesis also improve digestate stability and thus lower the environmental impact of the residues of AD. Compared to the conventional monodigester, two-stage AD improved the biostabilization efficiency of codigested FW and waste water sludge from 6.5% to 40.6% The application of digestate as fertilizer is facilitated [29].
The implementation of a separate hydrolysis–acidogenesis stage in AD allows the concurrent production of hydrogen, SCCA and methane. While sequential production of hydrogen and methane improves energy efficiency, there are also various other potentials downstream from a hydrogen-producing hydrolysis stage featuring photo-fermentation, power generation in a microbial electrolyzer or a biochemical stage for biopolymer production. Rising interest has evolved in the production of biohythane via two-stage AD, a hydrogen-enriched biogas with better quality, good caloric efficiency and enhanced combustion. The hydrogen proportion in biohythane varies from 10–30% v/v of hydrogen, but also a low proportion enhances the combustion properties of a biogas blend [30][31]. Simultaneous production makes the process a good biorefinery concept, being more sustainable and economically viable [31][32].
There are a few studies that actually compare the efficiency of a hydrolysis stage with another pretreatment option. Shahriari et al. evaluated AD of liquid and solid kitchen waste with and without microwave pretreatment in single- and two-stage AD. Two-stage AD showed higher yields for liquid kitchen waste, while the microwave pretreatment was more effective for solid waste. Regarding overall energy efficiency though, they concluded that the hydrolysis stage offers more benefits than microwave pretreatment [33]. Similarly a full-scale co-digestion biogas plant has been upgraded with a hydrolysis stage instead of using the former practice of ultrasonic pretreatment as it promised higher yields, higher OLR and lower HRT [34].
Anaerobic digestion with a separate hydrolysis stage is mostly conducted without any treatment except some shredding of bulk substrate. Stage separation widens possible applications of the whole process with the introduction of intermediary steps or recirculation. It was reported, that AD of acidic citrus waste, where low pH and the main component D-limonene (68–98%) are toxic for many microorganisms, is feasible using two-stage AD. pH control in the hydrolysis stage via effluent recirculation helps to relieve the toxic effects of D-limonene, which is mostly retained in the first stage or can be filtrated, thus lowering the inhibitory effect on the sensitive methanogens [35]. The potential to use the hydrolysis stage as a detoxification step was also explored. It was found that inhibitors built up during hydrothermal pretreatment of solid waste can be removed by acidogenic bacteria in the hydrolysis step [14]. Likewise, inhibitors from thermal or chemical pretreatment of lignocellulosic biomass are degradable in the first stage by certain microbes [36]. Biological detoxification was examined with various microorganism like Coniochaeta ligniaria that can metabolize furans, among others. The drawbacks of biological detoxification are long process times and the consumption of sugars [37]. Countermeasures to relieve problems with inhibitors comprise methods of feedstock engineering, detoxification steps and genetic or evolutionary engineering, which are either feedstock specific or require several process steps [36]. Integrating a microbial detoxification step in two-stage AD may be a promising method to combine the advantages of pretreatment and a two-stage AD.
The stable process within a two-stage AD provides a better basis for on-demand production of biogas. Biomass-derived energy has the potential to balance fluctuating natural power sources like solar and wind energy, as biomass is highly available and AD predictable [38][39]. By variation of substrate addition intervals or mass flow rate, the methane production can be controlled also within conventional AD systems, but problems might occur due to substrate overload and quick acidification [39]. Fluctuating OLR and shock loadings in the hydrolysis stage were proven to have no influence on the total specific final product yields [28][10]. Depending on the substrate, the reaction time of higher methane production lays within days or weeks in a single-stage AD. The two-stage system offers higher flexibility by different options. Firstly, higher hydrolysis rates enable faster changes of methane production as shown by Linke et al., who demonstrated daily differences of 50–60% methane production with variable feeding in a two-stage system [40]. Secondly, the SCCA-rich effluent of the hydrolysis stage can be stored easily and fed to the second stage for gas-production on demand. As the hydrolysate mainly consists of readily degradable material, methane production can be raised within hours [38][39]. On-demand production of biogas was also shown for the lignocellulosic substrate grass silage by operational changes in recirculation [41].
Overall, the optimization and control of microbial hydrolysis in a separate stage in anaerobic digestion offers many advantages and can reduce costs and environmental impact. More information on possible reactor configurations, process conditions and integration into biorefinery systems can be found in the Review of Menzel et al.[11].
This entry is adapted from the peer-reviewed paper 10.3390/en13215555
References
- A. Castellano-Hinojosa; Caterina Armato; C. Pozo; Alejandro Gonzalez-Martinez; Jesús González-López; New concepts in anaerobic digestion processes: recent advances and biological aspects. Applied Microbiology and Biotechnology 2018, 102, 5065-5076, 10.1007/s00253-018-9039-9.
- Dinh Pham Van; Takeshi Fujiwara; Bach Leu Tho; Pham Phu Song Toan; Giang Hoang Minh; A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environmental Engineering Research 2019, 25, 1-17, 10.4491/eer.2018.334.
- Publications, European Biogas Association . EBA. Retrieved 2020-11-16
- Karolina Kucharska; Piotr Rybarczyk; Iwona Hołowacz; Rafał Łukajtis; Marta Glinka; Marian Kamiński; Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937, 10.3390/molecules23112937.
- H. Carrère; Georgia Antonopoulou; Rim Affes; Fabiana Passos; Audrey Battimelli; Gerasimos Lyberatos; Ivet Ferrer; Review of feedstock pretreatment strategies for improved anaerobic digestion: From lab-scale research to full-scale application. Bioresource Technology 2016, 199, 386-397, 10.1016/j.biortech.2015.09.007.
- Ulysse Brémond; Raphaëlle De Buyer; Jean-Philippe Steyer; Nicolas Bernet; Hélène Carrere; Biological pretreatments of biomass for improving biogas production: an overview from lab scale to full-scale. Renewable and Sustainable Energy Reviews 2018, 90, 583-604, 10.1016/j.rser.2018.03.103.
- F. Petracchini; F. Liotta; V. Paolini; M. Perilli; D. Cerioni; F. Gallucci; M. Carnevale; A. Bencini; A novel pilot scale multistage semidry anaerobic digestion reactor to treat food waste and cow manure. International Journal of Environmental Science and Technology 2017, 15, 1999-2008, 10.1007/s13762-017-1572-z.
- Federico Micolucci; Marco Gottardo; P. Pavan; Cristina Cavinato; David Bolzonella; Pilot scale comparison of single and double-stage thermophilic anaerobic digestion of food waste. Journal of Cleaner Production 2018, 171, 1376-1385, 10.1016/j.jclepro.2017.10.080.
- Antonio Giuliano; L. Zanetti; F. Micolucci; C. Cavinato; Thermophilic two-phase anaerobic digestion of source-sorted organic fraction of municipal solid waste for bio-hythane production: effect of recirculation sludge on process stability and microbiology over a long-term pilot-scale experience. Water Science and Technology 2014, 69, 2200-2209, 10.2166/wst.2014.137.
- S.J. Grimberg; D. Hilderbrandt; M. Kinnunen; S. Rogers; Anaerobic digestion of food waste through the operation of a mesophilic two-phase pilot scale digester – Assessment of variable loadings on system performance. Bioresource Technology 2015, 178, 226-229, 10.1016/j.biortech.2014.09.001.
- Theresa Menzel; Peter Neubauer; Stefan Junne; Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555, 10.3390/en13215555.
- Jo E Burgess; Brett I Pletschke; Hydrolytic enzymes in sewage sludge treatment: A mini-review. Water SA 2018, 34, 343, 10.4314/wsa.v34i3.180627.
- Michael E. Himmel; Qi Xu; Yonghua Luo; Shi-You Ding; Raphael Lamed; Edward A Bayer; Microbial enzyme systems for biomass conversion: emerging paradigms. Biofuels 2010, 1, 323-341, 10.4155/bfs.09.25.
- Wei Li; Jianbin Guo; Huicai Cheng; Wei Wang; Renjie Dong; Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: Viability, performance and microbial community evaluation. Applied Energy 2017, 189, 613-622, 10.1016/j.apenergy.2016.12.101.
- Shilva Shrestha; Xavier Fonoll; Samir Kumar Khanal; Lutgarde Raskin; Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresource Technology 2017, 245, 1245-1257, 10.1016/j.biortech.2017.08.089.
- Xue Chen; Hairong Yuan; Dexun Zou; Yanping Liu; Baoning Zhu; Akiber Chufo; Muhammad Jaffar; X. J. Li; Improving biomethane yield by controlling fermentation type of acidogenic phase in two-phase anaerobic co-digestion of food waste and rice straw. Chemical Engineering Journal 2015, 273, 254-260, 10.1016/j.cej.2015.03.067.
- Heike Sträuber; Martina Schröder; Sabine Kleinsteuber; Metabolic and microbial community dynamics during the hydrolytic and acidogenic fermentation in a leach-bed process. Energy, Sustainability and Society 2012, 2, 13, 10.1186/2192-0567-2-13.
- Wee Shen Lee; Adeline Seak May Chua; Hak Koon Yeoh; Gek Cheng Ngoh; A review of the production and applications of waste-derived volatile fatty acids. Chemical Engineering Journal 2014, 235, 83-99, 10.1016/j.cej.2013.09.002.
- Marco Gottardo; F. Micolucci; D. Bolzonella; Hinrich Uellendahl; Francesco Valentino; Pilot scale fermentation coupled with anaerobic digestion of food waste - Effect of dynamic digestate recirculation. Renewable Energy 2017, 114, 455-463, 10.1016/j.renene.2017.07.047.
- Jonas Lindner; Simon Zielonka; Hans Oechsner; Andreas Lemmer; Is the continuous two-stage anaerobic digestion process well suited for all substrates?. Bioresource Technology 2016, 200, 470-476, 10.1016/j.biortech.2015.10.052.
- Jon Garcia-Aguirre; Enrique Aymerich; Jaime González-Mtnez. De Goñi; Myriam Esteban-Gutiérrez; Selective VFA production potential from organic waste streams: Assessing temperature and pH influence. Bioresource Technology 2017, 244, 1081-1088, 10.1016/j.biortech.2017.07.187.
- M.A. De La Rubia; F. Raposo; Bárbara Rincón; R. Borja; Evaluation of the hydrolytic–acidogenic step of a two-stage mesophilic anaerobic digestion process of sunflower oil cake. Bioresource Technology 2009, 100, 4133-4138, 10.1016/j.biortech.2009.04.001.
- Yu Qin; Atsushi Higashimori; Li-Jie Wu; Toshimasa Hojo; Kengo Kubota; Jun Cheng; Phase separation and microbial distribution in the hyperthermophilic-mesophilic-type temperature-phased anaerobic digestion (TPAD) of waste activated sludge (WAS). Bioresource Technology 2017, 245, 401-410, 10.1016/j.biortech.2017.08.124.
- Juana Fernández-Rodríguez; M. Pérez; Luis Isidoro Romero-García; Semicontinuous Temperature-Phased Anaerobic Digestion (TPAD) of Organic Fraction of Municipal Solid Waste (OFMSW). Comparison with single-stage processes. Chemical Engineering Journal 2016, 285, 409-416, 10.1016/j.cej.2015.10.027.
- Zhang Bo; He Pin-Jing; Performance assessment of two-stage anaerobic digestion of kitchen wastes. Environmental Technology 2013, 35, 1277-1285, 10.1080/09593330.2013.866169.
- Marta Kinnunen; Daniel Hilderbrandt; Stefan J Grimberg; Shane W Rogers; Sumona Mondal; Comparative study of methanogens in one- and two-stage anaerobic digester treating food waste. Renewable Agriculture and Food Systems 2014, 30, 515-523, 10.1017/s1742170514000350.
- Syeda Amber Hameed; Rumana Riffat; BaoQiang Li; Iffat Naz; Malik Badshah; Safia Ahmed; Naeem Ali; Microbial population dynamics in temperature‐phased anaerobic digestion of municipal wastewater sludge. Journal of Chemical Technology & Biotechnology 2019, 94, 1816-1831, 10.1002/jctb.5955.
- Biswabandhu Chatterjee; Debabrata Mazumder; Role of stage-separation in the ubiquitous development of Anaerobic Digestion of Organic Fraction of Municipal Solid Waste: A critical review. Renewable and Sustainable Energy Reviews 2019, 104, 439-469, 10.1016/j.rser.2019.01.026.
- Elena Albini; Isabella Pecorini; Giovanni Ferrara; Improvement of Digestate Stability Using Dark Fermentation and Anaerobic Digestion Processes. Energies 2019, 12, 3552, 10.3390/en12183552.
- David Bolzonella; Federico Battista; Cristina Cavinato; Marco Gottardo; Federico Micolucci; Gerasimos Lyberatos; Paolo Pavan; Recent developments in biohythane production from household food wastes: A review. Bioresource Technology 2018, 257, 311-319, 10.1016/j.biortech.2018.02.092.
- Shikha Dahiya; A. Naresh Kumar; J. Shanthi Sravan; Sulogna Chatterjee; OmPrakash Sarkar; S. Venkata Mohan; Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresource Technology 2018, 248, 2-12, 10.1016/j.biortech.2017.07.176.
- G. De Gioannis; A. Muntoni; Alessandra Polettini; Raffaella Pomi; Daniela Spiga; Energy recovery from one- and two-stage anaerobic digestion of food waste. Waste Management 2017, 68, 595-602, 10.1016/j.wasman.2017.06.013.
- Haleh Shahriari; Mostafa Warith; Mohamed Hamoda; Kevin Kennedy; Evaluation of single vs. staged mesophilic anaerobic digestion of kitchen waste with and without microwave pretreatment. Journal of Environmental Management 2013, 125, 74-84, 10.1016/j.jenvman.2013.03.042.
- Andreas Blank; Erhard Hoffmann; Upgrading of a co-digestion plant by implementation of a hydrolysis stage. Waste Management & Research: The Journal for a Sustainable Circular Economy 2011, 29, 1145-1152, 10.1177/0734242x11423954.
- Lukitawesa; Rachma Wikandari; Ria Millati; Mohammad J. Taherzadeh; Claes Niklasson; Effect of Effluent Recirculation on Biogas Production Using Two-stage Anaerobic Digestion of Citrus Waste. Molecules 2018, 23, 3380, 10.3390/molecules23123380.
- Leif J. Jönsson; Carlos Martín; Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresource Technology 2016, 199, 103-112, 10.1016/j.biortech.2015.10.009.
- Daehwan Kim; Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309, 10.3390/molecules23020309.
- Susanne Theuerl; Christiane Herrmann; Monika Heiermann; Philipp Grundmann; Niels Landwehr; Ulrich Kreidenweis; A. Prochnow; The Future Agricultural Biogas Plant in Germany: A Vision. Energies 2019, 12, 396, 10.3390/en12030396.
- Henning Hahn; Bernd Krautkremer; Kilian Hartmann; Michael Wachendorf; Review of concepts for a demand-driven biogas supply for flexible power generation. Renewable and Sustainable Energy Reviews 2014, 29, 383-393, 10.1016/j.rser.2013.08.085.
- Bernd Linke; Angela Rodríguez-Abalde; Carsten Jost; Andreas Krieg; Performance of a novel two-phase continuously fed leach bed reactor for demand-based biogas production from maize silage. Bioresource Technology 2015, 177, 34-40, 10.1016/j.biortech.2014.11.070.
- David M Wall; Emily E Allen; R. O'shea; P. O'kiely; Jerry D Murphy; Investigating two-phase digestion of grass silage for demand-driven biogas applications: Effect of particle size and rumen fluid addition. Renewable Energy 2016, 86, 1215-1223, 10.1016/j.renene.2015.09.049.