As the core component of all organs, the extracellular matrix (ECM) is an interlocking macromolecular meshwork of proteins, glycoproteins, and proteoglycans that provides mechanical support to cells and tissues. In cancer, the ECM can be remodelled in response to environmental cues, and it controls a plethora of cellular functions, including metabolism, cell polarity, migration, and proliferation, to sustain and support oncogenesis. The biophysical and biochemical properties of the ECM, such as its structural arrangement and being a reservoir for bioactive molecules, control several intra- and intercellular signalling pathways and induce cytoskeletal changes that alter cell shapes, behaviour, and viability.
- extracellular matrix
- composition
- cancer
- desmoplasia
- therapeutical targets
1. Introduction
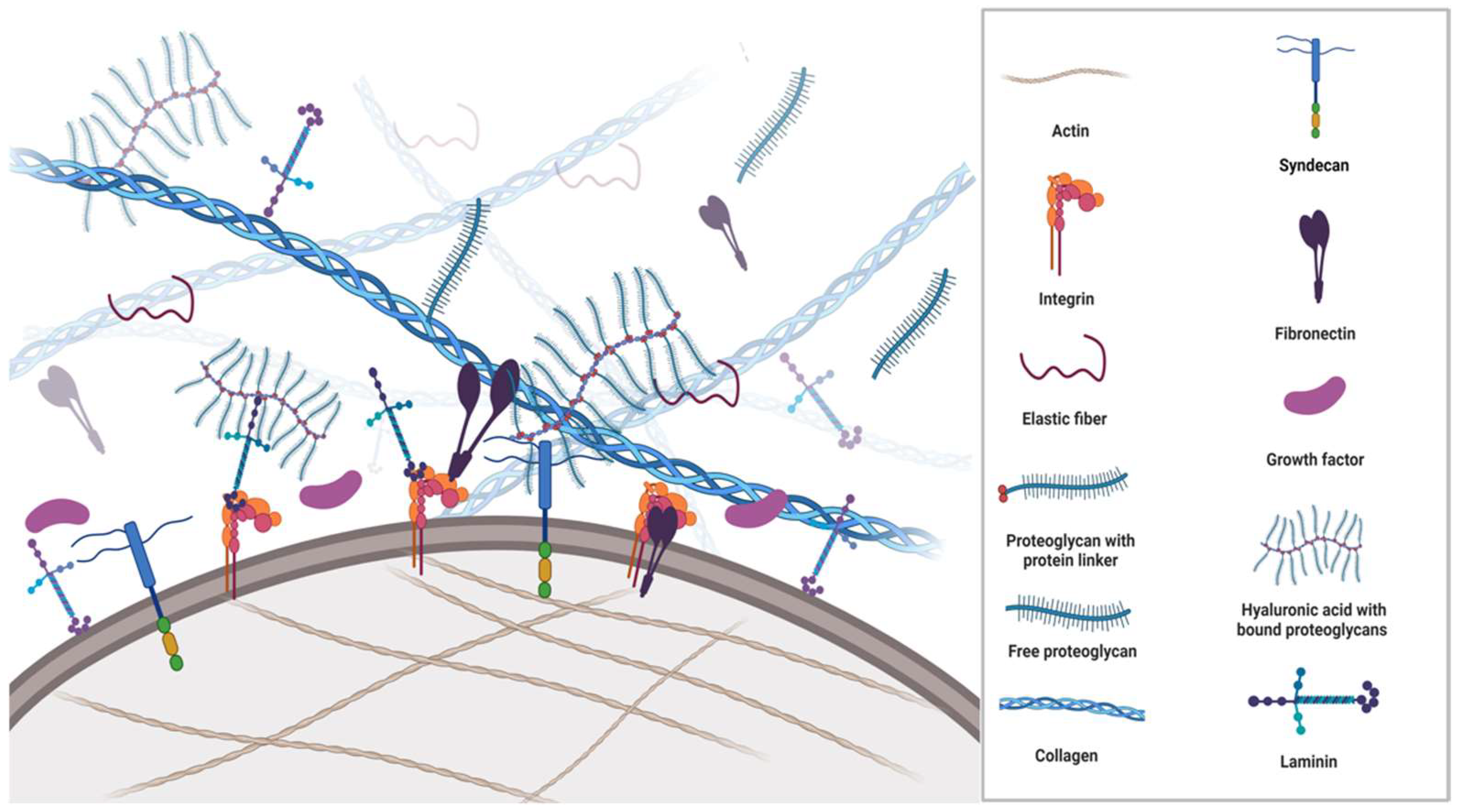
2. Components of ECM
3. Fibrous ECM Proteins
3.1. Collagen
3.2. Elastin
4. The Glycoproteins
4.1. Fibronectin
4.2. Laminin
| GF | ECM Protein | Growth Factor Functions |
|---|---|---|
| TGFβ | Type IV collagen Heparin/HS Fibronectin Fibrin/fibrinogen Betaglycan Decorin |
Modulates cell growth and differentiation. Stimulates the synthesis of collagen, fibronectin and other ECM components, including HA, TSP, and tenascin. Increases production of protease inhibitors. Reduces the synthesis and secretion of proteases. |
| HGF | Type I, III, IV, V, and VI collagen Heparin/HS Fibronectin Fibrin/fibrinogen |
Stimulates matrix remodelling and epithelial regeneration. Inhibits fibrosis. |
| IGF | IGF-binding protein | Stimulates cell mitogenesis, differentiation, and survival. Amplifies activity through the engagement of integrins or ECM glycosaminoglycans, heparin-binding domains, affecting cell adhesion and migration. |
| PDGF | SPARC Heparin/HS Fibronectin Fibrin/fibrinogen |
Regulates angiogenesis. Attracts fibroblasts and monocytes and accelerates granulation tissue formation and ECM deposition. |
| VEGF | Collagen Heparin/HS Fibronectin Fibrin/fibrinogen |
Controls blood vessel formation and growth. Binds to fibronectin to synergistically promote endothelial cell proliferation. |
| EGF | Collagen Fibronectin |
Stimulates epithelial cell proliferation. Regulates a subset of G1 cell-cycle events. Elevates levels of EGFR. |
| FGF | Heparin/HS Fibronectin Fibrin/fibrinogen |
Induces fibroblast proliferation and angiogenesis. Oligomerisation prolongs activity protection from proteolysis and endocytosis. |
5. Proteoglycans
Hyaluronic Acid
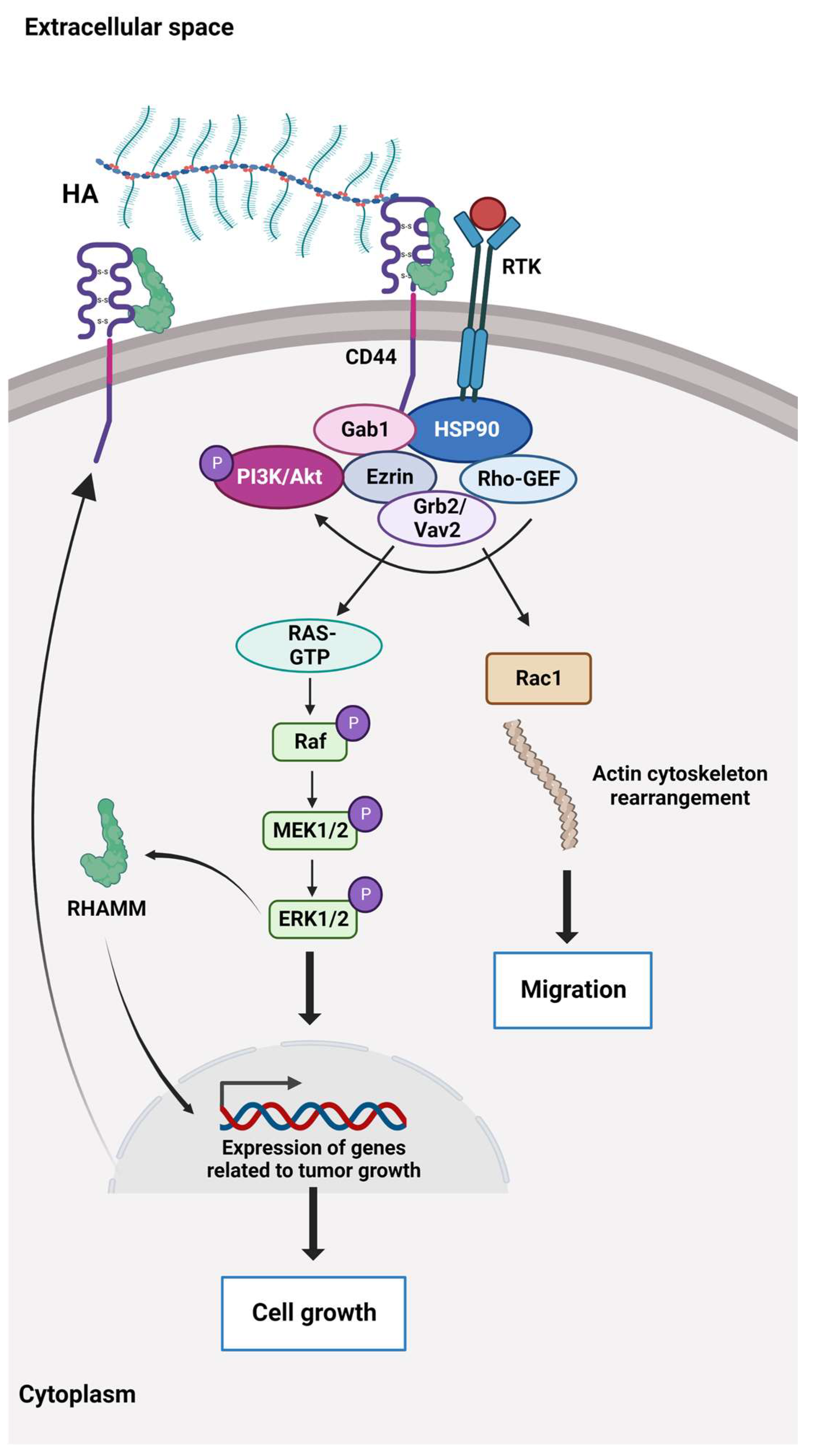
6. Sensing and Communication in ECM
| Signalling Pathways Affected by ECM Dysregulation | Breast Cancer | NSCLC | CRC | PDAC | Prostate Cancer | Ovarian Cancer | HCC | Glioblastoma | Malignant Melanoma |
|---|---|---|---|---|---|---|---|---|---|
| EMT | + | + | + | + | + | + | |||
| EGFR | + | + | + | ||||||
| FAK | + | + | + | ||||||
| FAK/Src | + | + | + | + | + | ||||
| HA | + | ||||||||
| Hedgehog | + | + | + | + | + | ||||
| Hippo | + | + | + | ||||||
| Hippo/YAP | + | ||||||||
| Integrin | + | + | + | + | + | + | + | + | |
| MAPK/ERK | + | + | + | + | |||||
| Notch | + | + | + | ||||||
| PI3K/AKT | + | + | + | + | + | + | + | + | + |
| RAS/RAF/MAPK/ERK | + | + | |||||||
| RhoA/ROCK | + | ||||||||
| Rho GTPase | + | ||||||||
| TGF-β | + | + | + | + | + | ||||
| TGF-β/Smad | + | + | + | ||||||
| Wnt/β-catenin | + | + | + | + | + | + | + | + |
7. ECM in Solid Cancers
This entry is adapted from the peer-reviewed paper 10.3390/cancers15072057
References
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
- Mishra, Y.G.; Manavathi, B. Focal adhesion dynamics in cellular function and disease. Cell. Signal. 2021, 85, 110046.
- Li, W.; Chi, N.; Rathnayake, R.A.C.; Wang, R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: A nanoscopic study. Biochem. Biophys. Res. Commun. 2021, 560, 66–71.
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the Tissues: Extracellular Matrix and Its Artificial Substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914.
- Song, D.; Shivers, J.L.; MacKintosh, F.C.; Patteson, A.E.; Janmey, P.A. Cell-induced confinement effects in soft tissue mechanics. J. Appl. Phys. 2021, 129, 140901.
- Müller, P.; Rogers, K.W.; Yu, S.R.; Brand, M.; Schier, A.F. Morphogen transport. Development 2013, 140, 1621–1638.
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492.
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120.
- Sun, X.; Wu, B.; Chiang, H.-C.; Deng, H.; Zhang, X.; Xiong, W.; Liu, J.; Rozeboom, A.M.; Harris, B.T.; Blommaert, E.; et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 2021, 599, 673–678.
- Wu, S.Z.; Roden, D.L.; Wang, C.; Holliday, H.; Harvey, K.; Cazet, A.S.; Murphy, K.J.; Pereira, B.; Al-Eryani, G.; Bartonicek, N.; et al. Stromal cell diversity associated with immune evasion in human triple-negative breast cancer. EMBO J. 2020, 39, e104063.
- Zhao, C.; Xiao, Y.; Ling, S.; Pei, Y.; Ren, J. Structure of Collagen. Methods Mol. Biol. 2021, 2347, 17–25.
- Antonio, J.D.; Jacenko, O.; Fertala, A.; Orgel, J.P. Collagen Structure-Function Mapping Informs Applications for Re-generative Medicine. Bioengineering 2020, 8, 3.
- Hsu, K.-S.; Dunleavey, J.M.; Szot, C.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Feng, Y.; Lutz, E.M.; Koogle, R.; et al. Cancer cell survival depends on collagen uptake into tumor-associated stroma. Nat. Commun. 2022, 13, 7078.
- Ma, W.; Ma, H.; Fogerty, F.J.; Mosher, D.F. Bivalent ligation of the collagen-binding modules of fibronectin by SFS, a non-anchored bacterial protein of streptococcus equi. J. Biol. Chem. 2015, 290, 4866–4876.
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, biomechanics and role of minor subtypes in cartilage. Nat. Rev. Mater. 2020, 5, 730–747.
- Burke, L.; Guterman, I.; Gallego, R.P.; Britton, R.G.; Burschowsky, D.; Tufarelli, C.; Rufini, A. The Janus-like role of proline metabolism in cancer. Cell Death Discov. 2020, 6, 104.
- Alcaide-Ruggiero, L.; Molina-Hernández, V.; Granados, M.M.; Domínguez, J.M. Main and Minor Types of Collagens in the Articular Cartilage: The Role of Collagens in Repair Tissue Evaluation in Chondral Defects. Int. J. Mol. Sci. 2021, 22, 13329.
- Kuivaniemi, H.; Tromp, G.; Prockop, D. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum. Mutat. 1999, 9, 300–331. Available online: https://onlinelibrary.wiley.com/doi/10.1002/(SICI)1098-1004(1997)9:4%3C300::AID-HUMU2%3E3.0.CO;2-9 (accessed on 13 February 2023).
- Hwang, S.J.; Ha, G.H.; Seo, W.Y.; Kim, C.K.; Kim, K.J.; Lee, S.B. Human collagen alpha-2 type I stimulates collagen synthesis, wound healing, and elastin production in normal human dermal fibroblasts (HDFs). BMB Rep. 2020, 53, 539–544.
- Wanjare, M.; Agarwal, N.; Gerecht, S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Physiol. 2015, 309, C271–C281.
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation: Not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205.
- Schmelzer, C.E.H.; Heinz, A.; Troilo, H.; Lockhart-Cairns, M.P.; Jowitt, T.A.; Marchand, M.F.; Bidault, L.; Bignon, M.; Hedtke, T.; Barret, A.; et al. Lysyl oxidase-like 2 (LOXL2)-mediated cross-linking of tropoelastin. FASEB J. 2019, 33, 5468–5481.
- Heinz, A. Elastases and elastokines: Elastin degradation and its significance in health and disease. Crit. Rev. Biochem. Mol. Biol. 2020, 55, 252–273.
- Shapiro, S.D.; Endicott, S.K.; Province, M.; Pierce, J.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834.
- Tembely, D.; Henry, A.; Vanalderwiert, L.; Toussaint, K.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Jaisson, S.; Galés, C.; Martiny, L.; et al. The Elastin Receptor Complex: An Emerging Therapeutic Target Against Age-Related Vascular Diseases. Front. Endocrinol. 2022, 13, 136.
- Thorlacius-Ussing, J.; Kehlet, S.N.; Rønnow, S.R.; Karsdal, M.A.; Willumsen, N. Non-invasive profiling of protease-specific elastin turnover in lung cancer: Biomarker potential. J. Cancer Res. Clin. Oncol. 2018, 145, 383–392.
- Li, J.; Xu, X.; Jiang, Y.; Hansbro, N.G.; Hansbro, P.M.; Xu, J.; Liu, G. Elastin is a key factor of tumour development in colorectal cancer. BMC Cancer 2020, 20, 217.
- Efthymiou, G.; Saint, A.; Ruff, M.; Rekad, Z.; Ciais, D.; van Obberghen-Schilling, E. Shaping Up the Tumour Microenvironment with Cellular Fibronectin. Front. Oncol. 2020, 10, 641.
- Cai, Q.; Yan, L.; Xu, Y. Anoikis resistance is a critical feature of highly aggressive ovarian cancer cells. Oncogene 2014, 34, 3315–3324.
- Patankar, M.; Eskelinen, S.; Tuomisto, A.; Karttunen, T.J. KRAS and BRAF mutations induce anoikis resistance and characteristic 3D phenotypes in Caco-2 cells. Mol. Med. Rep. 2019, 20, 4634–4644.
- Yu, Y.; Liu, B.; Li, X.; Lu, D.; Yang, L.; Chen, L.; Li, Y.; Cheng, L.; Lv, F.; Zhang, P.; et al. ATF4/CEMIP/PKCα promotes anoikis resistance by enhancing protective autophagy in prostate cancer cells. Cell Death Dis. 2022, 13, 46.
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-κB. Oncogene 2006, 25, 4341–4349.
- Gee, E.P.S.; Yüksel, D.; Stultz, C.M.; Ingber, D.E. SLLISWD sequence in the 10FNIII domain initiates fibronectin fibrillogenesis. J. Biol. Chem. 2013, 288, 21329–21340.
- Ohashi, T.; Kiehart, D.P.; Erickson, H.P. Dynamics and elasticity of the fibronectin matrix in living cell culture visualized by fibronectin-green fluorescent protein. Proc. Natl. Acad. Sci. USA 1999, 96, 2153–2158.
- Paten, J.A.; Martin, C.L.; Wanis, J.T.; Siadat, S.M.; Figueroa-Navedo, A.M.; Ruberti, J.W.; Deravi, L.F. Molecular Interactions between Collagen and Fibronectin: A Reciprocal Relationship that Regulates De Novo Fibrillogenesis. Chem 2019, 5, 2126–2145.
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028.
- Griggs, L.A.; Hassan, N.T.; Malik, R.S.; Griffin, B.P.; Martinez, B.A.; Elmore, L.W.; Lemmon, C.A. Fibronectin fibrils regulate TGF-β1-induced Epithelial-Mesenchymal Transition. Matrix Biol. 2017, 60–61, 157–175.
- Bae, Y.K.; Kim, A.; Kim, M.K.; Choi, J.E.; Kang, S.H.; Lee, S.J. Fibronectin expression in carcinoma cells correlates with tumour aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum. Pathol. 2013, 44, 2028–2037.
- Wang, J.; Li, R.; Li, M.; Wang, C. Fibronectin and colorectal cancer: Signaling pathways and clinical implications. J. Recept. Signal Transduct. 2020, 41, 313–320.
- Fang, T.; Lv, H.; Lv, G.; Li, T.; Wang, C.; Han, Q.; Yu, L.; Su, B.; Guo, L.; Huang, S.; et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat. Commun. 2018, 9, 191.
- Joseph, J.V.; Conroy, S.; Pavlov, K.; Sontakke, P.; Tomar, T.; Eggens-Meijer, E.; Balasubramaniyan, V.; Wagemakers, M.; Dunnen, W.F.D.; Kruyt, F.A. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α–ZEB1 axis. Cancer Lett. 2015, 359, 107–116.
- Ryu, M.H.; Park, H.M.; Chung, J.; Lee, C.H.; Park, H.R. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma in-vasion via upregulation of alpha5 integrin and fibronectin. Biochem. Biophys. Res. Commun. 2010, 393, 11–15.
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250–263.
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587.
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2018, 75–76, 12–26.
- Ishihara, J.; Ishihara, A.; Fukunaga, K.; Sasaki, K.; White, M.J.V.; Briquez, P.S.; Hubbell, J.A. Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing. Nat. Commun. 2018, 9, 2163.
- Islam, K.; Thummarati, P.; Kaewkong, P.; Sripa, B.; Suthiphongchai, T. Role of laminin and cognate receptors in cholangiocarcinoma cell migration. Cell Adhes. Migr. 2021, 15, 152–165.
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55.
- Gopal, S.; Amran, A.; Elton, A.; Ng, L.; Pocock, R. A somatic proteoglycan controls Notch-directed germ cell fate. Nat. Commun. 2021, 12, 6708.
- Czarnowski, D. Syndecans in cancer: A review of function, expression, prognostic value, and therapeutic significance. Cancer Treat. Res. Commun. 2021, 27, 100312.
- Scott, J. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J. 1992, 6, 2639–2645.
- Lin, W.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606.
- Lodish, H.; Berk, A.; Matsudaira, P.; Kaiser, C. Molecular Cell Biology, 5th ed.; W.H Freeman: New York, NY, USA, 2003.
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525.
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18.
- Enemark, M.B.; Hybel, T.E.; Madsen, C.; Lauridsen, K.L.; Honoré, B.; Plesner, T.L.; Hamilton-Dutoit, S.; d’Amore, F.; Ludvigsen, M. Tumour-Tissue Expression of the Hyaluronic Acid Receptor RHAMM Predicts Histological Transformation in Follicular Lymphoma Patients. Cancers 2022, 14, 1316.
- Auvinen, P.; Tammi, R.; Kosma, V.-M.; Sironen, R.; Soini, Y.; Mannermaa, A.; Tumelius, R.; Uljas, E.; Tammi, M. Increased hyaluronan content and stromal cell CD44 associate with HER2 positivity and poor prognosis in human breast cancer. Int. J. Cancer 2012, 132, 531–539.
- Michalczyk, M.; Humeniuk, E.; Adamczuk, G.; Korga-Plewko, A. Hyaluronic Acid as a Modern Approach in Anticancer Therapy-Review. Int. J. Mol. Sci. 2022, 24, 103.
- Kim, P.K.; Halbrook, C.J.; Kerk, S.A.; Radyk, M.; Wisner, S.; Kremer, D.M.; Sajjakulnukit, P.; Andren, A.; Hou, S.W.; Trivedi, A.; et al. Hyaluronic acid fuels pancreatic cancer cell growth. eLife 2021, 10, e62645.
- Zhang, G.; Guo, L.; Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Gao, F. A novel role of breast cancer-derived hyaluronan on inducement of M2-like tumour-associated macrophages formation. Oncoimmunology 2016, 5, e1172154.
- Kuang, D.M.; Wu, Y.; Chen, N.; Cheng, J.; Zhuang, S.M.; Zheng, L. Tumour-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood 2007, 110, 587–595.
- Wu, W.; Chen, L.; Wang, Y.; Jin, J.; Xie, X.; Zhang, J. Hyaluronic acid predicts poor prognosis in breast cancer patients: A protocol for systematic review and meta analysis. Medicine 2020, 99, e20438.
- Klichinsky, M.; Ruella, M.; Shestova, O.; Lu, X.M.; Best, A.; Zeeman, M.; Schmierer, M.; Gabrusiewicz, K.; Anderson, N.R.; Petty, N.E.; et al. Human chimeric antigen receptor macrophages for cancer immuno-therapy. Nat. Biotechnol. 2020, 38, 947–953.
- Wang, S.; Yang, Y.; Ma, P.; Zha, Y.; Zhang, J.; Lei, A.; Li, N. CAR-macrophage: An extensive immune enhancer to fight cancer. Ebiomedicine 2022, 76, 103873.
- Chen, X.; Zhu, H.; Feng, X.Q.; Li, X.; Lu, Y.; Wang, Z.; Rezgui, Y. Predictive assembling model reveals the self-adaptive elastic properties of lamellipodial actin networks for cell migration. Commun. Biol. 2020, 3, 616.
- Grandy, C.; Port, F.; Pfeil, J.; Gottschalk, K.-E. Influence of ROCK Pathway Manipulation on the Actin Cytoskeleton Height. Cells 2022, 11, 430.
- Northey, J.J.; Przybyla, L.; Weaver, V.M. Tissue force programs cell fate and tumour aggression. Cancer Discov. 2017, 7, 1224–1237.
- Han, S.J.; Azarova, E.V.; Whitewood, A.J.; Bachir, A.; Guttierrez, E.; Groisman, A.; Horwitz, A.R.; Goult, B.T.; Dean, K.M.; Danuser, G.; et al. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. eLife 2021, 10, e66151.
- Seetharaman, S.; Vianay, B.; Roca, V.; Farrugia, A.J.; De Pascalis, C.; Boëda, B.; Dingli, F.; Loew, D.; Vassilopoulos, S.; Bershadsky, A.; et al. Microtubules tune mechanosensitive cell responses. Nat. Mater. 2021, 21, 366–377.
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82.
- Yamashiro, Y.; Thang, B.Q.; Ramirez, K.; Shin, S.J.; Kohata, T.; Ohata, S.; Nguyen, T.A.V.; Ohtsuki, S.; Nagayama, K.; Yanagisawa, H. Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. USA 2020, 117, 9896–9905.
- Pocaterra, A.; Romani, P.; Dupont, S. YAP/TAZ functions and their regulation at a glance. J. Cell Sci. 2020, 133, jcs230425.
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53.
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2021, 21, 60–78.
- Zhang, P.; Wang, J.; Luo, W.; Yuan, J.; Cui, C.; Guo, L.; Wu, C. Kindlin-2 Acts as a Key Mediator of Lung Fibroblast Activation and Pulmonary Fibrosis Progression. Am. J. Respir. Cell Mol. Biol. 2021, 65, 54–69.
- Guo, L.; Cui, C.; Zhang, K.; Wang, J.; Wang, Y.; Lu, Y.; Chen, K.; Yuan, J.; Xiao, G.; Tang, B.; et al. Kindlin-2 links mechano-environment to proline synthesis and tumour growth. Nat. Commun. 2019, 10, 845.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix Metalloproteinases: Regulators of the Tumour Microenvironment. Cell 2010, 141, 52–67.
- Silsirivanit, A. Chapter Five: Glycosylation markers in cancer. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 189–213.
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Tumour Vasculature: Improving Drug Delivery and Efficacy. Trends Cancer 2018, 4, 258–259.
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238.
- Miskolczi, Z.; Smith, M.; Rowling, E.J.; Ferguson, J.; Barriuso, J.; Wellbrock, C. Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene 2018, 37, 3166–3182.
- Ajeti, V.; Nadiarnykh, O.; Ponik, S.M.; Keely, P.J.; Eliceiri, K.W.; Campagnola, P.J. Structural changes in mixed Col I/Col V collagen gels probed by SHG microscopy: Implications for probing stromal alterations in human breast cancer. Biomed. Opt. Express 2011, 2, 2307–2316.
- Kauppila, S.; Stenbäck, F.; Risteli, J.; Jukkola, A.; Risteli, L. Aberrant type I and type III collagen gene expression in human breast cancer in vivo. J. Pathol. 1999, 186, 262–268.
- Kamei, M.; Saunders, W.B.; Bayless, K.J.; Dye, L.; Davis, G.E.; Weinstein, B.M. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 2006, 442, 453–456.
- Hielscher, A.; Ellis, K.; Qiu, C.; Porterfield, J.; Gerecht, S. Fibronectin Deposition Participates in Extracellular Matrix Assembly and Vascular Morphogenesis. PLoS ONE 2016, 11, e0147600.
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.S.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumour microenvironment: Colon tumour-derived extracellular matrix promotes angiogenesis and tumour cell growth. Biomaterials 2017, 116, 118–129.
- Ruehle, M.A.; Eastburn, E.A.; LaBelle, S.A.; Krishnan, L.; Weiss, J.A.; Boerckel, J.D.; Wood, L.B.; Guldberg, R.E.; Willett, N.J. Extracellular matrix compression temporally regulates microvascular angiogenesis. Sci. Adv. 2020, 6, eabb6351. Available online: https://www.science.org/doi/10.1126/sciadv.abb6351 (accessed on 21 November 2022).
- Daub, J.T.; Merks, R.M.H. A Cell-Based Model of Extracellular-Matrix-Guided Endothelial Cell Migration During Angiogenesis. Bull. Math. Biol. 2013, 75, 1377–1399.
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307.
- Muranen, T.; Iwanicki, M.P.; Curry, N.L.; Hwang, J.; DuBois, C.D.; Coloff, J.L.; Hitchcock, D.S.; Clish, C.B.; Brugge, J.S.; Kalaany, N.Y. Starved epithelial cells uptake extracellular matrix for survival. Nat. Commun. 2017, 8, 13989.
- Rainero, E.; Howe, J.D.; Caswell, P.T.; Jamieson, N.B.; Anderson, K.; Critchley, D.R.; Machesky, L.; Norman, J.C. Ligand-Occupied Integrin Internalization Links Nutrient Signaling to Invasive Migration. Cell Rep. 2015, 10, 398–413.
- Kim, S.G.; Hoffman, G.R.; Poulogiannis, G.; Buel, G.R.; Jang, Y.J.; Lee, K.W.; Kim, B.Y.; Erikson, R.L.; Cantley, L.C.; Choo, A.Y.; et al. Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol. Cell 2012, 49, 172–185.
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976.
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumour Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239.
- Korolchuk, V.I.; Saiki, S.; Lichtenberg, M.; Siddiqi, F.H.; Roberts, E.A.; Imarisio, S.; Jahreiss, L.; Sarkar, S.; Futter, M.; Menzies, F.M.; et al. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011, 13, 453–460.
- Muranen, T.; Selfors, L.M.; Worster, D.T.; Iwanicki, M.P.; Song, L.; Morales, F.C.; Gao, S.; Mills, G.B.; Brugge, J.S. Inhibition of PI3K/mTOR Leads to Adaptive Resistance in Matrix-Attached Cancer Cells. Cancer Cell 2012, 21, 227–239.
- Koorman, T.; Jansen, K.A.; Khalil, A.; Haughton, P.D.; Visser, D.; Rätze, M.A.K.; Haakma, W.E.; Sakalauskaitè, G.; van Diest, P.J.; de Rooij, J.; et al. Spatial collagen stiffening promotes collective breast cancer cell invasion by reinforcing extracellular matrix alignment. Oncogene 2022, 41, 2458–2469.
- Esfahani, P.; Levine, H.; Mukherjee, M.; Sun, B. Three-dimensional cancer cell migration directed by dual mechanochemical guidance. Phys. Rev. Res. 2022, 4, L022007.
- Han, W.; Chen, S.; Yuan, W.; Fan, Q.; Tian, J.; Wang, X.; Liu, R.; Qu, J. Oriented collagen fibers direct tumour cell intravasation. Proc. Natl. Acad. Sci. USA 2016, 113, 11208–11213.
- Piotrowski-Daspit, A.S.; Nerger, B.A.; Wolf, A.E.; Sundaresan, S.; Nelson, C.M. Dynamics of Tissue-Induced Alignment of Fibrous Extracellular Matrix. Biophys. J. 2017, 113, 702–713.
- Anguiano, M.; Morales, X.; Castilla, C.; Pena, A.R.; Ederra, C.; Martínez, M.; Ariz, M.; Esparza, M.; Amaveda, H.; Mora, M.; et al. The use of mixed collagen-Matrigel matrices of increasing complexity recapitulates the biphasic role of cell adhesion in cancer cell migration: ECM sensing, remodeling and forces at the leading edge of cancer invasion. PLoS ONE 2020, 15, e0220019.
- Gaggar, A.; Weathington, N. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Investig. 2016, 126, 3176–3184.
- He, X.; Lee, B.; Jiang, Y. Extracellular matrix in cancer progression and therapy. Med. Rev. 2022, 2, 125–139.
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219.
- Olabi, S.; Ucar, A.; Brennan, K.; Streuli, C.H. Integrin-Rac signalling for mammary epithelial stem cell self-renewal 06 Biological Sciences 0601 Biochemistry and Cell Biology. Breast Cancer Res. 2018, 20, 128.
- Wei, S.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.; et al. Matrix stiffness drives epithelial–mesenchymal transition and tumour metastasis through a TWIST1–G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688.
- Barrera, L.N.; Ridley, P.M.; Bermejo-Rodriguez, C.; Costello, E.; Perez-Mancera, P.A. The role of microRNAs in the modulation of cancer-associated fibroblasts activity during pancreatic cancer pathogenesis. J. Physiol. Biochem. 2022, 79, 193–204.
