Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
Chronic heart failure (HF) is classified into two types: HFrEF—heart failure with reduced ejection fraction; and HFpEF—heart failure with “preserved” EF. In both the cases, increased left ventricular filling pressure impact on symptoms and on myocardial perfusion. Thus, device-based solutions were proposed to improve cardiac physiology in HF and to prevent or treat post-capillary pulmonary hypertension.
- heart failure
- pediatric heart failure
- pharmacotherapy for heart failure
1. Background
Heart failure is a complex and progressive clinical and pathophysiological syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood [1].
Chronic heart failure (HF) is classified into two types: HFrEF—heart failure with reduced ejection fraction; and HFpEF—heart failure with “preserved” EF.
HFrEF occurs with inadequate ventricular contraction and is commonly associated with a dilated left ventricle. HFpEF primarily depends on diastolic dysfunction and increased ventricular filling pressures, leading to elevations in atrial and venous pressures and, in the case of LV dysfunction, an increase in pulmonary arterial pressure. Right heart failure (RVF) is not common in children but could be associated with several congenital heart diseases (CHD), including Tetralogy of Fallot, transposition of great arteries, atrial septal defects, Ebstein anomaly, arrhythmogenic right ventricular cardiomyopathy (ARVCM) and right ventricle dysfunction in single ventricle physiology [2]. The great variability of causes and different pathophysiological mechanisms generally lead to the development of the same symptoms: jugular venous distention, hepatomegaly, and occasionally peripheral edema and ascites [3].
Pediatric HF differs from adult HF, mainly due to the variety of etiologies classified into three main categories: heart failure due to cardiomyopathy, heart failure due to CHD with biventricular or univentricular physiology, and heart failure from acquired heart disease [4]. The prevalence of CHD is around 0.8% of live births, and about 70% of them are at risk of HF. Fortunately, surgical or percutaneous treatment can effectively resolve or prevent the development of heart failure in those patients. Despite all, few papers are focused on the epidemiology of HF in CHD; the prevalence of CHD in severe HF ranged between 25 and 75%, with large geographical variations. Congenital heart diseases, such as an anomalous left coronary artery from the pulmonary artery (ALCAPA), critical aortic stenosis, coarctation of the aorta, and single-ventricle physiology, are most frequently associated with HF in newborns and infants [5]. Dilated cardiomyopathy (DCM) is a leading cause of HF in older children and is one of the main indications for pediatric heart transplantations. The incidence is 0.58–0.73 per 1,000,000 children/year; it is more common in adolescents, with an incidence of 4.4 to 4.8 per 100,000 [6] (Table 1).
Table 1. Main causes of pediatric heart failure.
| Category | Diagnoses in Category |
|---|---|
| Congenital heart disease (CHD) | An anomalous left coronary artery from the pulmonary artery (ALCAPA), critical aortic stenosis, coarctation of the aorta, and single-ventricle congenital heart disease. |
| Inherited cardiomyopathy | HCM, RCM, DCM, ARVC, LVNC, fatty acid oxidation disorder mitochondrial disorders, Barth syndrome, Danon disease, and limb-girdle dystrophy. |
| Acquired conditions | Myocarditis, Kawasaki disease, arrhythmia, systemic lupus erythematosus, dermatomyositis, rheumatic heart disease, and chemotherapy. |
HCM—hypertrophic cardiomyopathy; RCM—restrictive cardiomyopathy; DCM—dilated cardiomyopathy; ARVC—right ventricle arrhythmogenic cardiomyopathy; LVNC—left ventricular noncompaction cardiomyopathy.
The diagnosis of HF is an integration of clinical signs, symptoms, and instrumental investigations. A chest X-ray and EKG can help determine whether nonspecific signs such as tachypnea, dyspnea, and failure to thrive are attributable to heart failure. On the chest radiograph, the cardiac shadow may be enlarged, and in some cases, signs of increased pulmonary vascular texture, alveolar edema, and pleural effusion may be noted. The EKG commonly shows nonspecific abnormalities such as ventricular hypertrophy or repolarization abnormalities, or some patients can present a rhythm alteration (supraventricular tachycardia, atrial fibrillation, atrial flutter, atrioventricular block, and ventricular tachycardia). Laboratory markers are a useful tool to establish the diagnosis of HF. The most important are BNP (atrial natriuretic peptide) and pro-BNP (N-terminal protein of BNP); those markers are often elevated in children with HF and may represent poor prognostic factors [7]. It has demonstrated a strong correlation between increased NT-pro-BNP and the worst symptoms and decreased ventricular function [8]. Electrolytes and renal function may be impaired, especially in patients with acute decompensation. Hyponatremia is frequently identified in patients with advanced disease and is associated with worse outcomes in hospitalized patients, which is why it is recommended to monitor renal function in these patients to prevent renal insufficiency [9]. Echocardiography remains the fundamental exam for the diagnosis of heart failure. In general, echocardiography allows for the evaluation of the dilatation of the cardiac chambers, the systolic or diastolic dysfunction, and any associated cardiac defects. It is mandatory to rule out coronary abnormalities in infants with a first finding of dilated cardiomyopathy to exclude ALCAPA [4].
Heart failure remains a challenge for pediatric cardiologists: most of the recommendations for chronic pediatric HF management were extrapolated from adult heart failure trials, despite the fact that the etiopathology of the two groups is different. Thus, the development of novel therapeutic approaches for the treatment of this disorder is crucial. In addition, few data are available on the epidemiology and clinical outcome of heart failure in the pediatric population, mostly focused on the hospitalization rate. A retrospective analysis showed that hospitalizations for cardiomyopathies and heart failure in children were significantly longer than in adults. The overall mortality rate was higher for pediatric patients [10], in particular when HF was associated with CHDs (>20% in this subgroup) [11].
2. Interventional Cardiology
2.1. Device Therapy
Device-based solutions to improve cardiac physiology in HF and to prevent or treat post-capillary pulmonary hypertension have been proposed in the adult population. For example, left ventricular expanders, mechanical circulatory support devices, and neurostimulators are used in the adult population, but there is no experience with this device in pediatric HF. The only option currently used in pediatric populations is an inter-atrial device.
The creation of an inter-atrial shunt is advisable in several cardiovascular diseases, especially in HF. Increased left ventricular (LV) filling pressure and left atrial pressure are observed both in HFpEF and in HFrEF. Diminished relaxation can be due to a variety of causes, including secondary LV hypertrophy or restrictive cardiomyopathies. In addition, mitral valve regurgitation can further contribute to increasing LA pressure. A long-term increase in LA pressure determines progressive lung congestion and type II pulmonary hypertension. In addition, high LV filling pressures impact myocardial perfusion, rhythm abnormalities, and mechanical desynchrony. Thus, the creation of an interatrial shunt allows to reduce the left atrial pressure and left ventricular filling pressure. In addition, the LA shunt might reduce the LV volume overload, increasing the responsiveness to the pharmacologic therapy. Finally, RV overload might stiffen the interventricular septum and reduce septal dyskinesia.
In the pediatric population, stent implantation into the interatrial septum or balloon dilatation of a pre-existing atrial communication or after a septal puncture has been used, especially in patients with complex heart anatomy, to improve symptoms [33]. These methods present some limitations: balloon dilatation often results in early reclosure and is thus effective in acute settings; in addition, the ASD size cannot be calibrated. A stent implantation is highly effective to create an ASD with a pre-fixed diameter; however, stent placement might be challenging, and the removal needs a surgical approach [34].
New inter-atrial devices, such as the Inter Atrial Shunt Device (IASD, Corvia Medical, Tewksbury, MA, USA), the V-Wave (Caesarea, Israel), and the Atrial Flow Regulator (AFR, Occlutech, Schaffhausen, Switzerland) interatrial shunt device, have been used to create a permanent controlled left-to-right shunting in adult patients [35,36]. Those devices have not been used in children yet, except for the AFR.
The AFR is a self-expandable, small implantable device made of Nitinol (Figure 1). It is similar to a self-centering atrial septal defect occluder device with a 4 to 10 mm hole in the middle (in Europe, only 8 and 10 mm obtained the CE mark). The device can be implanted, through a trans-venous approach, into an existing atrial septal defect/patent foramen ovale or after a trans-septal puncture and pre-dilatation with a 6 to 10 mm balloon catheter. The goal of this therapy is to maintain stable and pre-fixed interatrial communication in order to reduce the interatrial gradient to 5–8 mmHg. In clinical practice, reduced diastolic filling pressure leads to reduced symptoms, improved exercise tolerance, and a higher quality of life.
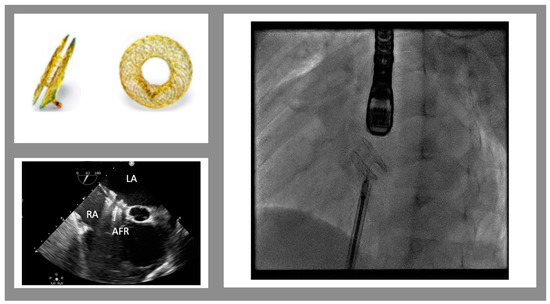
Figure 1. Atrial flow regulator device. Top on the left: the device in frontal and lateral views. The bottom on the left shows the trans-esophageal view of the implanted device (mid-esophageal view, 52°). On the right is a fluoroscopic view of the device just before the release. (LA left atrium, RA right atrium, AFR atrial flow regulator)
The AFR is being evaluated for adult heart failure in two clinical studies (PRELIEVE and PROLONGER), in a prospective registry (AFteR Follow-up Study to Monitor the Efficacy and Safety of the Occlutech AFR in Heart Failure Patients), and in an ongoing clinical study for pulmonary arterial hypertension (PROPHET Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator in Patients With Pulmonary Hypertension). The prospective, non-randomized, multicenter, phase 2 PRELIEVE study reported the first-in-human use of the AFR for older patients with HFpEF (LVEF >40%; n 24) or HFrEF (LVEF 15–39%; n 29). The resting PAWP decreased by 5 mm Hg at 3 months after the AFR implantation. No complication occurred, in particular no shunt occlusion, stroke, or new right HF was observed during the 1-year follow-up period, with clinical improvements in certain patients [36,37].
In the pediatric population, some cases describe the safety and feasibility of the procedure in patients with PAH [38], Fontan’s failing circulation [39,40], and in young children [41]. In a recent multicenter US experience, AFR was implanted in six pediatric patients, five of whom had Fontan circulation failure and only one had left ventricular hypertension. All pediatric patients survived without complications. Oxygen saturations increased in all Fontan patients and were maintained through a follow-up of 8 weeks; also, NYHA class improved from a median of stage 3 before the procedure to a median of stage 2 after the procedure [39]. Currently, AFR in the pediatric population has been more frequently used for PAH and Fontan failure than heart failure compared to adults. However, a clinical case series with 3 patients (6 to 13 years old) affected by restrictive cardiomyopathy and undergoing AFR implantation to reduce LA volume overload and PAH postcapillary has been reported. The procedure improved LA dilation, postcapillary pulmonary hypertension, and HF symptoms in all 3 patients [42]. In another retrospective, single-center analysis, five pediatric patients with symptomatic heart failure not responding to maximal medical therapy and with a history of complex congenital or acquired cardiac diseases underwent AFR implantation. The procedure was successfully completed in all five patients. In one case, a hybrid procedure was performed during pulmonary artery surgical banding with a trans-atrial approach through the right atrial free wall. Clinical improvement was observed in all patients with a reduction of LA dilation, postcapillary pulmonary hypertension, and HF symptoms [43]. Follow-up studies are required to monitor the efficacy and safety of the Occlutech AFR and to identify the category of patients that can benefit most.
2.2. Cardiac Resynchronization Therapy
Cardiac resynchronization therapy is known worldwide (since 2009 American Heart Association guidelines on HF) to be useful for patients affected by HfrEF and LVEF < 35%. CRT is recommended in adult patients affected by HF with reduced ejection fraction (LVEF ≤ 35%), LV dilatation, and NHYA classes II, III, and IV despite maximization of drug therapy and duration of QRS > 120 ms. This therapy demonstrated a slower progression of heart failure due to the improvement of cardiac motion and a reduced risk of sudden death with a general improvement in quality of life [44].
According to the 2013 EHRA and AEPC pediatric guidelines, CRT might be used in pediatrics, especially for adolescents or young adults, with some clarifications. In pediatric and CHD populations, the QRS duration cut-off is the 98th percentile for age. In patients with specific progressive forms of DCM (ventricular non-compaction, neuromuscular, and mitochondrial disease), CRT should be considered carefully and individually because of the lack of evidence on efficacy. Patients with systemic ventricles could benefit from CRT, but with a specific evaluation of the mechanical dyssynchrony [45]. Some pediatric patients with a history of severe left ventricular dysfunction (LVEF < 30%) associated or not with ventricular arrhythmias secondary to CMD or CHD (valvular stenosis type aortic or mitral valve) may benefit from defibrillator-associated cardiac resynchronization therapy (CRT-D).
This entry is adapted from the peer-reviewed paper 10.3390/jcm12072611
This entry is offline, you can click here to edit this entry!
